| Table of contents |

|
| Characteristics of p block elements |

|
| p block elements electronic configuration |

|
| Properties of p block elements |

|
| Uses of p block elements |

|
The p block elements consist of elements where the valence electron occupies the p-subshell of the outermost energy level. This block is located on the far right side of the periodic table. There are three p orbitals, allowing a maximum of six electrons to occupy them. Consequently, the periodic table includes six groups of p-block elements: Group 13 (IIIA), Group 14 (IVA), Group 15 (VA), Group 16 (VIA), Group 17 (VIIA), and Group 18 (zero). The electronic configuration of p block elements follows the pattern ns2np1-6, with the exception of Helium.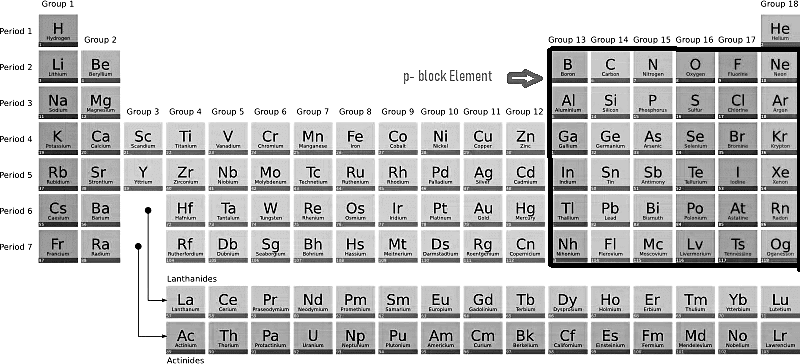
Characteristics of p block elements
- Variety of Elements: The p-block elements encompass metals, non-metals, and metalloids.
- Covalent Compound Formation: They predominantly form covalent compounds.
- Conductivity: The p-block elements often possess a shiny appearance and display good electrical and thermal conductivity.
- Ionization Enthalpy: Compared to s-block elements, p-block elements have significantly higher ionization enthalpies.
- Flame Color: These elements do not impart a distinct color to a flame.
- Acidic Oxides: They primarily form acidic oxides.
- Electronegativity: Electronegativity decreases from top to bottom within a group, and increases from left to right across a period.
- Reducing Property: The reducing property decreases from left to right within a period, but increases from top to bottom within a group.
- Non-Metallic Character: Non-metallic character steadily increases from left to right across a period, but decreases from top to bottom within a group.
- Maximum Oxidation State: The total number of valence electrons, comprising the ns and np electrons, determines the highest oxidation state possible for a p-block element. Moving from left to right in the p-block, the number of potential oxidation states generally increases.
- Allotropy: Several elements in the p-block series, such as arsenic, carbon, silicon, phosphorus, sulfur, boron, germanium, and tin, exhibit the phenomenon of allotropy.
- Catenation Property: Carbon, silicon, germanium, nitrogen, oxygen, sulfur, and other elements in the p-block series demonstrate the property of catenation, allowing them to form chains or rings of atoms.
p block elements electronic configuration
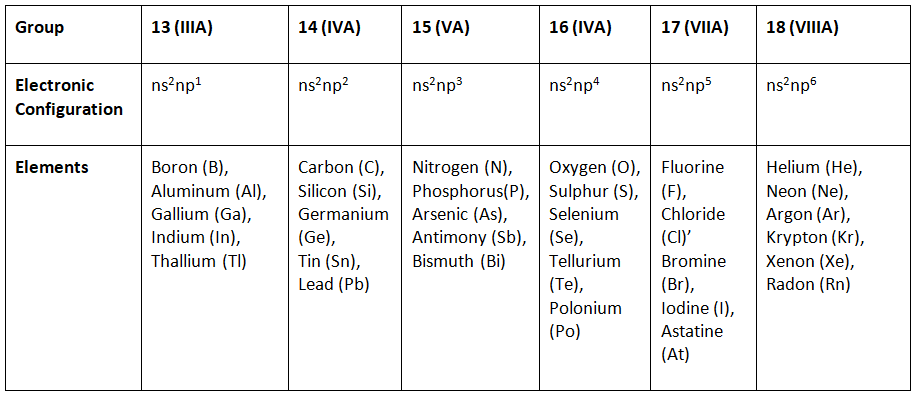
Properties of p block elements
The differences in an element’s inner core have a significant impact on both its chemical and physical properties, including atomic and ionic radii, ionization enthalpy, and several others. The characteristics of the elements in a group of p-block are thus shown to vary significantly.
The initial p-block element is different from the other elements in two key ways:
- The size and any other characteristics that depend on size come first.
- The second distinction only affects the p-block element, which is created as a result of the interactions between d-orbitals and the valence shells of heavier elements.
Uses of p block elements
- Germanium, silicon, arsenic, and gallium are all employed as semiconductors.
- Alum is extensively used for water purification.
- The glass and pottery industries use the boron compound borax.
- Steel’s hardness is increased with boron.
- Numerous applications include the usage of carbon and its compounds.

|
Explore Courses for JEE exam
|

|
















