Separation of Substances Class 6 Worksheet Science Chapter 3
Q1: True or False
i. The property used in separating a mixture of two solids by winnowing is the difference in weight.
ii. A mixture of milk and water can be separated by filtration.
iii. A mixture of powdered salt and sugar can be separated by the process of winnowing.
iv. Separation of sugar from tea can be done with filtration. v. Grain and husk can be separated with the process of decantation.
v. Grain and husk can be separated with the process of decantation.
vi. Sieving is used when the components of the mixture are of different sizes.
vii. The method of filtration is also used in the process of preparing cottage cheese in homes.
viii. When no more salt can be dissolved in the given amount of water at a particular temperature, the solution is said to be unsaturated.
ix. Centrifugation is used to separate cream from curd.
x. Hand-picking can be used to separate cashew nuts from a mixture of almonds and cashew nuts.
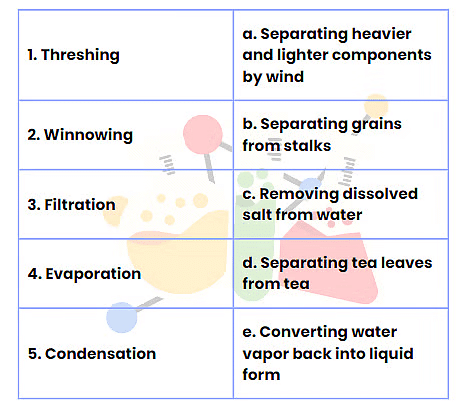
Q2: Match the Following
Q3: Fill in the Blanks
i. The process of separating a liquid from solid sediment is called .
ii. The method of separating seeds of paddy from its stalks is called .
iii. When milk, cooled after boiling, is poured onto a piece of cloth the cream (malai) is left behind on it. This process of separating cream from milk is an example of .
iv. Salt is obtained from seawater by the process of .
v. is used to remove impurities and bran from the flour.
vi. The two liquids that do not mix with each other can be separated by .
vii. We see water drops under the plate that has been used to cover a container containing milk that has just been boiled. This is due to process of .

Assertion and Reason Questions
Q4: Assertion (A): The process of conversion of liquid water to its vapours by heating the liquid is called evaporation.
Reason (R): The process of conversion of water vapours to liquid by cooling the vapours is called condensation.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Q5: Assertion (A): The process of settling of heavier insoluble particles from a suspension of a substance in water known as decantation.
Reason (R): This process along with sedimentation is used to get clear water from muddy water.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
Answer the following
Q6: Write any two methods used for separation of substances.
Q7: How can we separate sand from water?
Q8: What happens when you continue to add salt to a fixed amount of water, and how can you increase the amount of salt that dissolves in water after it becomes saturated?
Q9: Which method would you prefer to separate solid dissolved in liquid?
Q10: Filtration method is used to separate tea leaves from prepared tea. Which other method can be used?
Q11: List various methods of separation of components from their mixtures.
Q12: The process of adding alum to water to fasten sedimentation is called loading. Why has this name been given to the process?
Q13: How can we separate oil and water from their mixture?
Q14: Why are we able to dissolve more solute in a solvent at high temperature?
Q15: Why sieving is not used to separate very small stones from rice grains?
Q16: There are 3 beakers half filled with water. Now add 3 spoons of sugar in the first beaker, 5 spoons of sugar in the second beaker and 7 spoons of sugar in the third beaker. Stir the solution of all three beakers. Which solution is more saturated?
Q17: What is winnowing? Where is it used?
Q18: Why do we separate substances?
Q19: How will you separate husk or dirt particles from a given sample of pulses before cooking?
Q20: What is sieving? Where is it used?
You can access the solutions to this worksheet here.
FAQs on Separation of Substances Class 6 Worksheet Science Chapter 3
| 1. How can we separate a mixture of salt and water? |  |
| 2. What method can be used to separate a mixture of sand and iron filings? |  |
| 3. Can filtration be used to separate a mixture of salt and water? |  |
| 4. How can we separate a mixture of oil and water? |  |
| 5. Is distillation an effective method for separating a mixture of alcohol and water? |  |






















