Class 9 Science Chapter 4 Practice Question Answers - Structure of the Atom
Multiple Choice Questions
Q1: Maximum number of electrons present in 'N' shell is
(a) 18
(b) 32
(c) 2
(d) 8
Ans: (b)
N -shell ⇒ n = 4
We know that, maximum number of electrons present in an orbit = 2n2
No of electrons 'N ' shell = 2 × 42
= 2 × 16 = 32
Q2: In 1932. J. Chadwick discovered another sub-atomic particle which had no charge and a mass nearly equal to that of a proton. It was eventually named as
(a) proton
(b) neutron
(c) electron
(d) α-particle
Ans: (b)
Neutrons have no charge and mass nearly equal to that of protons.
Q3: Which of the following is a property of isotopes?
(a) They have the same number of electrons.
(b) They have different numbers of protons.
(c) They have different chemical properties.
(d) They have the same mass number.
Ans: (a)
They have the same number of protons. They have same chemical properties. They have different mass numbers.
Q4: Valency of oxygen is
(a) 1
(b) 2
(c) 3
(d) 4
Ans: (b)
Electronic configuration is O is 2, 6.
Hence, The valency of oxygen = 8 - 6 = 2
Q5: In the nucleus of 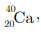 , there are
, there are
(a) 40 protons and 20 electrons
(b) 20 protons and 40 electrons
(c) 20 protons and 20 neutrons
(d) 20 protons and 40 neutrons
Ans: (c)
Atomic number of calcium (Z) = 20
No. of protons = No. of electrons = 20
Mass number of calcium (A) = 40
No. of neutrons (n) = A - Z
= 40 - 20 = 20
Therefore, there are 20 electrons, 20 protons and 20 neutrons in calcium.
Q6: The number of neutrons in the element 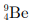 is
is
(a) 4
(b) 5
(c) 9
(d) 13
Ans : (b)
Number of neutrons
= Mass number - Atomic number
= 9 - 4 = 5
Q7: Which of the following elements contains only two electrons in the outermost shell?
(a) Helium
(b) Beryllium
(c) Magnesium
(d) All of these
Ans: (d)
All have two electrons in valence shell.
Q8: The charge on the atom having 17 protons, 18 electrons is
(a) +1
(b) -1
(c) -2
(d) zero
Ans : (b)
No. of electrons > No. of protons
Q9: Proton was discovered by
(a) Thomson
(b) Rutherford
(c) Chadwick
(d) Goldstein
Ans: (d)
E. Goldstein discovered the presence of new radiations in a gas discharge and called them canal rays. These rays were positively charged radiations which ultimately led to the discovery of sub-atomic particle-proton.
Q10: Which of the following is a property of isotopes?
(a) They have the same number of electrons.
(b) They have different numbers of protons.
(c) They have different chemical properties.
(d) They have the same mass number.
Ans : (a)
They have the same number of protons. They have same chemical properties. They have different mass numbers.
Q11: Isotopes have
(a) same physical and chemical properties
(b) same physical properties but different chemical properties
(c) same chemical properties but different physical properties
(d) different physical and chemical properties
Ans: (c)
Chemical properties of an element depend on its number of electrons, and the isotopes have same number of electrons. Thus, they show similar chemical properties.
Physical properties depends on mass number and isotopes have different mass numbers. Thus, they show different physical properties.
Q12: The part of an atom where nearly whole mass is concentrated is called
(a) extra-nuclear part
(b) nucleus
(c) atom
(d) neutron
Ans : (b)
The entire mass of the atom is concentrated in the nucleus.
Q13: Which of the following elements has same number of protons, electrons and neutrons?
(a) Al
(b) Mg
(c) P
(d) Cl
Ans: (b)
Mg is represented as 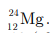 It has protons, electrons and neutrons equal to 12 (all are same).
It has protons, electrons and neutrons equal to 12 (all are same).
Q14: Atom X and atom Y have similar chemical properties. If the proton number of atom X is 12, What is the likely proton number of atom Y ?
(a) 5
(b) 10
(c) 14
(d) 20
Ans : (d)
Electronic configuration of atom X is 2, 8, 2. Since atom X and atom Y have similar chemical properties, atom Y must have same number of valence electrons as atom X . So, the proton number of atom Y is 10 and electronic configuration is 2, 8, 8, 2.
Q15: The ion of an element has 2 positive charge. Mass number of the atom is 24 and the number of neutrons is 12. What is the number of electrons in the ion?
(a) 8
(b) 10
(c) 12
(d) 24
Ans : (b)
The ion of an element has 2 positive charges. A = 24, n = 12, p = 24 - 12 = 12, e = 12 - 2 = 10
So, the number of electrons in the ion = 10.
Fill in the blanks.
Q16: The subatomic particle not present in a hydrogen atom is .........
Ans : Neutron
In a hydrogen atom, the subatomic particle not present is the "Neutron." This is because a hydrogen atom, in its most basic form, only contains one proton and one electron. Neutrons are typically present in the nucleus of atoms, but hydrogen is unique in that its nucleus contains only a single proton.
Q17: Almost all the mass of an atom is concentrated in a small region of space called the .........
Ans : Nucleus
Almost all the mass of an atom is concentrated in a small region of space called the "Nucleus". The nucleus of an atom contains protons and neutrons, which collectively account for nearly all the atom's mass. Electrons, which orbit the nucleus, contribute very little to the atom's overall mass due to their significantly smaller size.
Q18: The number of neutrons in the nucleus of an atom can be calculated by ......... the atomic number ........ its mass number.
Ans : eight
The number of neutrons in the nucleus of an atom can be calculated by "subtracting" the atomic number "from" its mass number. Unfortunately, the provided answer "eight" is incorrect. The atomic number of an atom is equal to the number of protons it contains, while the mass number is the total number of protons and neutrons. By subtracting the atomic number from the mass number, you can find the number of neutrons.
Q19: An atom of an element has 11 protons, 11 electrons and 12 neutrons. The atomic mass of the atom is .........
Ans : 23
An atom of an element that has 11 protons, 11 electrons, and 12 neutrons has an atomic mass of "23". This is calculated by adding the number of protons and neutrons in the nucleus of the atom. Since protons and neutrons each have a mass of approximately 1 atomic mass unit, the total atomic mass is 11 (protons) + 12 (neutrons) = 23.
Q20: Cathode rays are a beam of fast moving ..........
Ans : electrons
"Electrons" are the particles that make up Cathode rays. These rays are a stream of electrons observed in vacuum tubes. When a high voltage is applied, electrons are emitted from the cathode and form a beam of fast-moving particles, which is why they are referred to as cathode rays.
True/False
Q21: α -particles are same thing as helium atoms.
Ans : True
α-particles are indeed the same thing as helium atoms. This is because an α-particle is composed of two protons and two neutrons, which is exactly the same composition as a helium nucleus. The only difference is that the α-particle does not have any electrons, which are present in a helium atom.
Q22: An electron has a mass that is much less than a proton.
Ans : True
An electron is much lighter than a proton. The mass of a proton is approximately 1836 times the mass of an electron. Therefore, the statement that an electron has a mass much less than a proton is true.
Q23: Atoms of an element may have more or less neutrons or electrons than other atoms of the same element.
Ans : True
Atoms of the same element can have different numbers of neutrons and electrons. Atoms with the same number of protons but different numbers of neutrons are called isotopes. Also, an atom may gain or lose electrons to achieve stability and in the process become an ion. Therefore, it is true that atoms of an element can have more or less neutrons or electrons than other atoms of the same element.
Q24: Thomson proposed that the nucleus of an atom contains protons and neutrons.
Ans : False
It was not J.J Thomson but Ernest Rutherford who proposed the concept of the nucleus and its composition. In his model, he stated that the nucleus is the central part of the atom and it contains protons and neutrons. Thomson, in his plum pudding model, proposed that electrons are embedded in a positive sphere.
Q25: There is no particle of matter smaller than an atom.
Ans : False
There are particles smaller than an atom. Atoms are made up of subatomic particles - protons, neutrons and electrons. Later, with the advancement of science and technology, even smaller particles like quarks, leptons etc. were discovered.
Matching Questions
Direction : In the section, each question has two matching lists. Choices for the correct combination of elements from List-I and List-II are given as options (a), (b), (c) and (d) out of which one is correct.
Q26: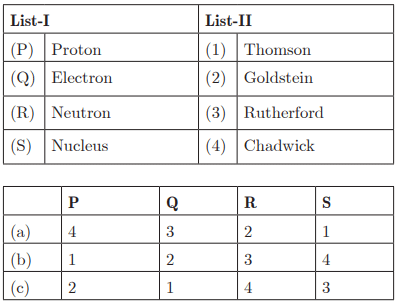
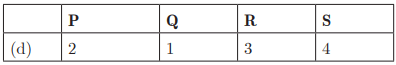 Ans: (c) P - 2, Q - 1, R - 4, S - 3
Ans: (c) P - 2, Q - 1, R - 4, S - 3
Q27: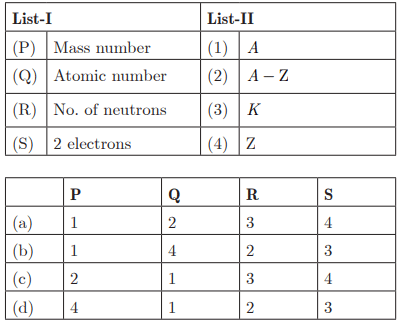 Ans: (b) P - 1, Q - 4, R - 2, S - 3
Ans: (b) P - 1, Q - 4, R - 2, S - 3
Q28: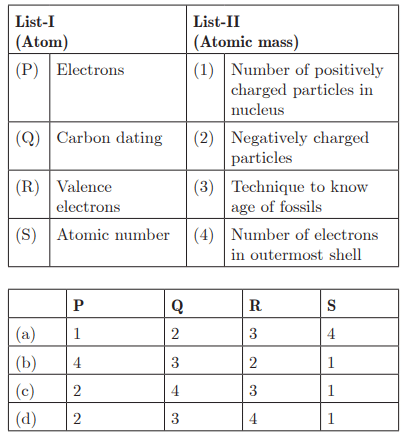
Ans: (d) P - 2, Q - 3, R - 4, S - 1
Q29: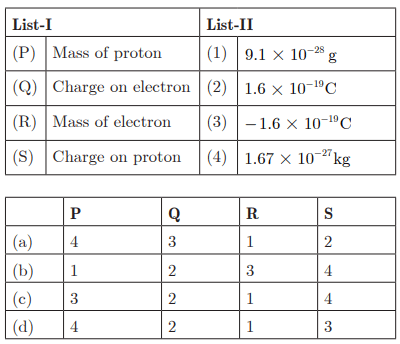
Ans : (a) P - 4, Q - 3, R - 1, S - 2
Q30: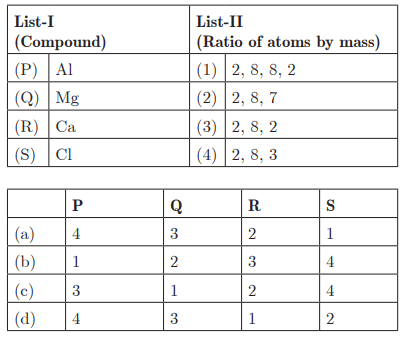
Ans : (d) P - 4, Q - 3, R - 1, S - 2
|
84 videos|541 docs|60 tests
|
















