Class 9 Science Chapter 4 HOTS Questions - Structure of the Atom
Q1: Both helium (He) and beryllium (Be) have two valence electrons. Whereas He represents a noble gas element, Be does not. Assign reason.
Ans: The element He (Z = 2) has two electrons present in the only shell i.e., K-shell. Since this shell can have a maximum of two electrons only therefore, He is a noble gas element. The element Be (Z = 4) has electronic configuration as : 2, 2. Although the second shell has also two electrons but it is not complete. It can still accommodate six more electrons. Therefore, the element beryllium does not represent a noble gas element.
Q2: Which of the two will be chemically more reactive ; element X with atomic number 17 or element Y with atomic number 16 ?
Ans: The electronic configuration of the two elements are as follows :
X(Z = 16): K (2), L(8), M(6) ;
Y(Z = 17): K(2), L(8), M(7)
The element X needs two electrons in the M shell to have the noble gas configuration of element, Ar (Z = 18). However, the element Y needs only one electron to achieve this. This means that the element Y has a greater urge or desire to take up one electron from an outside atom. It is therefore, more reactive than the element X which needs two electrons.
Q3: An atom of an element has three electrons in the third shell which is the outermost shell. Write
- the electronic configuration
- the atomic number
- number of protons
- valency
- the name of the element
- its nature whether metal or non-metal.
Ans: The third shell is M shell. If the atom of the element has three electrons in the third shell, this means that K and L shells are already filled.
- Electronic configuration : 2, 8, 3.
- Atomic number = No. of electrons =13
- Number of protons = No. of electrons =13
- Valency of the element = 3
- The element with Z = 13 is aluminium (Al)
- It is a metal.
Q4: Study the data given below and answer the questions which follow :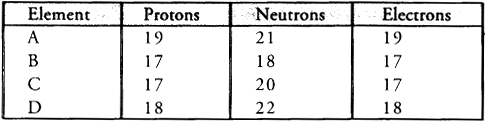
- Write the mass number and atomic number of the particles A, B, C and D.
- Which represent a pair of isotopes ?
Ans:
- Particle A : Mass number = 7 ; Atomic number = 3
- Particle B : Mass number = 17 ; Atomic number = 9
- Particle C : Mass number =16, Atomic number = 8
- Particle D : Mass number =18, Atomic number = 8
- Particles C and D represent a pair of isotopes since they have same atomic number.
Q5: The number of protons, neutrons and electrons in particles from A to E are given below: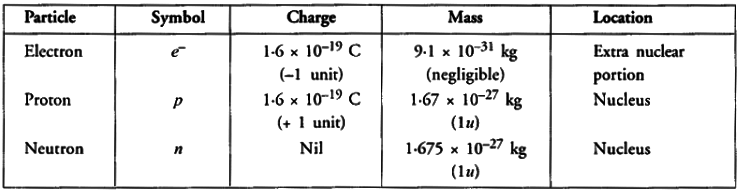
- Which one is a cation ?
- Which one is an anion ?
- Which represent pair of isotopes ?
Ans:
- B is a monovalent cation (B+)
- E is a monovalent anion (E–)
- A and D represent pair of isotopes.
Q6: One electron is present in the outermost shell of the atom of an element ‘Z’.
(a) What will be the nature of this element?
(b) What will be the value of charge of the ion formed, if this electron is removed from the outermost shell?
Ans:
(a) Element ‘Z’ will be a metal because it has only one electron in the outermost shell, so it is electropositive.
(b) After loss of one electron, ‘Z’ will acquire one positive charge.
Z → Z+ + 1 e–
Q7: An atom ‘M’ of an element reacts with oxygen to form M2O3. Calculate the valency of the element ‘M’.
Ans: Two atoms of element ‘M’ combine with 3 atoms of oxygen.
∴ Number of oxygen atoms combining with one atom of element ‘M’ = 3/2
Therefore, the valency of element ‘M’ 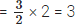
Q8: Explain why chlorine, whether as the element or its compounds, always has relative atomic mass of about 35.5.
Ans: The relative atomic mass is the average mass of one of the atoms and has to take into account the relative abundances of the various isotopes.
Natural chlorine always contains about 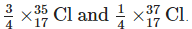
Therefore, relative atomic mass of chlorine 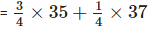
= 35.5 u
Q9: How many electrons will weigh 1 g?
Ans: Mass of an electron = 9.11 × 10-31 kg
∴ Mass of 9.11 × 10-31 kg = 1 electron
Now, mass of 1g, i.e., 10-31 kg will have 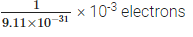
= 1.098 × 1027 electrons.
Q10: Composition of the nuclei of two atomic species ‘X’ and ‘Y’ are given below: Give the mass number of ‘X’ and ‘Y’. What is the relationship between the two species?
Give the mass number of ‘X’ and ‘Y’. What is the relationship between the two species?
Ans: (i) Atomic mass of element ‘X’ = Number of protons + Number of neutrons
= 8 + 8 = 16 u
(ii) Atomic mass of element ‘Y’ = Number of neutrons + Number of protons
= 10 + 8 = 18 u
Relationship between X and Y: The atomic number of both the elements is same but their atomic masses are different. Hence,they are isotopes of each other.
Q11: Complete the following gaps in the given table: Ans: We know that the number of protons = Atomic number
Ans: We know that the number of protons = Atomic number
Number of protons = Number of electrons
Mass number = Number of protons + number of neutrons
Using these relationships, we can fill up these gaps as follows: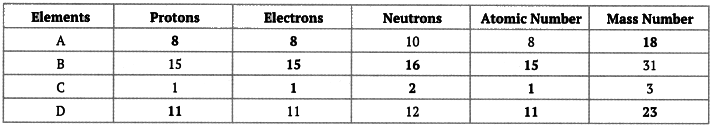
Q12: An element ‘X’ has mass number 4 and atomic number 2. Write the valency of this element. Will it react with other atoms of different elements?
Ans: We know that only valence electrons take part in bond formation with different atoms. In the atom of ‘X’ element there are only two electrons since atomic number is 2. Thus, K shell is fully filled for this atom. Hence, its valency is zero. It will not react with other atoms of different elements.
|
84 videos|478 docs|60 tests
|
















