Pollination & Fertilization | Botany Optional for UPSC PDF Download
Introduction
It is essential to recall that haploid pollen grains and egg cells are produced through meiosis. Following the formation of gametophytes, the subsequent crucial stages of sexual reproduction, namely, pollination and fertilization, come into play. These processes lead to the formation of a zygote, which eventually develops into an embryo. However, before fertilization can occur, pollen must be transferred from the stamen to the stigma of the carpel. This transfer of pollen is known as pollination, and it can be facilitated by various agents such as wind, water, or animals.
One distinctive feature of flowering plants is the phenomenon of double fertilization, which occurs subsequent to pollination. In this unit, we will explore various aspects related to pollination, including its types, the important adaptations plants have evolved to ensure successful pollination, the structural characteristics of the pistil, interactions between pollen and pistil, the intricacies of double fertilization, issues of incompatibility, and the phenomenon of apomixis.
Objectives
Upon completing this unit, you should be able to:
- Explain the process of sexual reproduction in flowering plants.
- Describe the mechanisms employed by plants to disperse pollen grains effectively for pollination.
- Understand why hybridization fails in certain cases.
- Define methods to overcome issues of incompatibility in plant reproduction.
- Analyze how apomixis functions to ensure the survival of specific plant species.
- Explore the control mechanisms that plants have developed to prevent indiscriminate sexual reproduction.
Pollination
Pollination is the process of transferring pollen from the dehiscing anthers to the pistil. Unlike animals, plants cannot move to find mates for sexual reproduction. Therefore, they rely on external agents or agencies for the transfer of pollen grains from the male parent to the stigma of the female parent. In some exceptional cases, such as Vallisneria, an aquatic plant, complete male flowers may be transported to female flowers.
Various physical (wind and water) and biological (insects, birds, and bats) agents facilitate cross-pollination. The key requirements for pollination are the dehiscence of anthers and the transfer of pollen.
Anther Dehiscence: Anther dehiscence refers to the release of pollen grains from dry and mature anthers. It involves the rupture of the anther wall due to mechanical pressure generated by the fibrous thickenings of endothecial cells along the stomium (the region where the mechanical layer does not differentiate). In cases where endothecium is absent, the mechanical role is assumed by epidermal cells. In most angiosperms, the stomium is a narrow strip along the entire length of the anther lobe, but it can also be restricted to a lid or valve (e.g., Berberidaceae) or pores (e.g., Solanum, Cassia, Polygala).
Pollen Transfer: Pollen transfer can be either autogamous (self-pollination), where pollen grains from an anther reach the stigma of the same flower, or cross-pollination, where pollen from one plant reaches the pistil of another plant of the same species. When pollination occurs between two flowers of the same plant, it is called geitonogamy, and if it happens between two flowers on different plants, it is termed xenogamy.
Types of Pollination
- Self-Pollination: This involves the transfer of pollen grains from the anther to the stigma of the same flower. In chasmogamous flowers, mature anthers and stigma are exposed to pollinating agents. In cleistogamous flowers, fertilization occurs without exposing the sex organs to the atmosphere.
- Cross-Pollination: In this type, pollen from the anther of one individual is transferred to the stigma of another individual of the same species. This process is facilitated by physical or biological agents such as wind, water, insects, birds, or mammals. While cross-pollination is essential in unisexual flowers, bisexual flowers may have adaptations to prevent self-pollination, including self-sterility, dichogamy, herkogamy, and heterostyly.
- Anemophily: Also known as wind pollination, anemophily involves the transport of pollen grains through wind currents. Anemophilous plants produce numerous small, dry, lightweight, and smooth pollen grains that are released on warm and dry days. These plants have unisexual flowers with reduced sepals and petals, allowing for effective positioning of the long and feathery stigma for pollen interception. Examples include palms, grasses, millets, and bamboos.
- Hydrophily: Not all hydrophytes (aquatic plants) are pollinated by water. Many aquatic plants are anemophilous or entomophilous. Hydrophilous plants often have highly reduced or absent floral envelopes. Hydrophily can involve underwater pollination (hyphydrophily) or pollination at the water's surface (ephydrophily). Vallisneria is an example of ephydrophily, where submerged male flowers float on the water surface and come in contact with pistillate flowers for pollination.
- Entomophily: This type of pollination relies on insects, such as bees and butterflies, to carry pollen. Insects perceive flower colors differently from humans, as they can see in the ultraviolet range. Flowers pollinated by insects often have yellow or blue petals and may have ultraviolet markings that guide insects to nectar or pollen sources. Some flowers use a "fly-trap mechanism" by emitting unpleasant odors to attract specific pollinators.
- Ornithophily: In tropical regions, birds, including hummingbirds, sunbirds, and honeyeaters, are important pollinators. Bird-pollinated flowers are typically red, orange, or yellow, as birds see well in this range. These flowers often lack strong scents since birds do not have a strong sense of smell. Characteristic features of ornithophilous flowers include tubular, cup-shaped, or urn-shaped forms, bright colors, an abundance of pollen and nectar. Hummingbirds can hover over pendant flowers, while sunbirds can perch on erect flowers.
- Cheiropterophily: Bats are important pollinators in tropical regions. Bat-pollinated flowers are typically dusky and dull-colored, and they produce a strong scent, often resembling fermented fruit. Bats are attracted to the scent and lap up nectar, transferring pollen as they visit different flowers. To facilitate bat pollination, these flowers are often borne singly or in clusters away from branches and foliage.
Self- vs Cross-Pollination
- Self-pollination and cross-pollination represent two different methods of pollen transfer in plants, each with its own advantages and disadvantages.
Self-Pollination
- Advantages:
- Self-pollination is highly certain, ensuring successful reproduction.
- It can occur in the absence of external pollinators, making it a reliable method.
- Self-pollination can be advantageous when environmental conditions are stable and predictable.
- Disadvantages:
- Over many generations, self-pollination can result in weaker offspring due to inbreeding depression.
- It lacks genetic recombination, limiting genetic diversity.
- From an evolutionary perspective, self-pollination is a disadvantage because it doesn't introduce genetic variability.
Cross-Pollination
- Advantages:
- Cross-pollination brings pollen from genetically different plants, promoting genetic heterogeneity.
- Offspring produced through cross-pollination tend to be more vigorous and better adapted to diverse environmental conditions.
- Cross-pollinated species have a wider distribution compared to self-pollinated species, which can be advantageous for evolution.
- Disadvantages:
- Cross-pollination is uncertain because it depends on external pollinators.
- It requires a significant expenditure of resources by plants to produce a large amount of pollen to account for potential wastage.
- When animals serve as pollinators, plants must provide rewards like pollen or nectar, which can be costly.
To encourage cross-pollination and prevent self-pollination, many flowering plants have evolved various mechanisms and adaptations, including:
- Dichogamy: In some species, the anthers and stigma mature at different times, preventing self-pollination. For example, in sunflowers, anthers dehisce before the stigma becomes receptive (protandry), while in Mirabilis and Magnolia, the stigma becomes receptive before anther dehiscence (protogyny).
- Herkogamy: Certain species have structural adaptations that prevent pollen from coming into contact with the stigma of the same flower. These adaptations ensure that self-pollination cannot occur. For instance, some plants have stigmas that project beyond the level of anthers, preventing the pollen from landing on the stigma. Orchids and Calotropis have pollinia (pollen in sacs) that cannot reach the stigma of the same flower.
- Self-Sterility: In many species, self-pollination does not lead to fertilization because pollen germination or pollen tube growth on the stigma is inhibited. Therefore, pollen from another plant is required for effective fertilization. Self-sterility is widespread in flowering plants and is genetically controlled.
- Dicliny: Some species have unisexual flowers, where male and female flowers are either on the same plant (monoecious) or different plants (dioecious). Pollination in these species involves two different flowers, making it a form of cross-pollination.
These mechanisms and adaptations have evolved in response to the specific benefits of cross-pollination, which enhances genetic diversity and contributes to the evolutionary success of flowering plants.
Fertilization
The ultimate goal of pollination in flowering plants is to achieve successful fertilization, which involves the fusion of male and female gametes. However, there are several barriers and interactions that must be overcome for this process to be successful, beginning with pollen-stigma interaction.
Pollen-Stigma Interaction
- The Stigma: After landing on the stigma, a pollen grain germinates and produces a pollen tube, which carries the male gametes. Stigmas can be classified into two main types based on the presence or absence of stigmatic exudate at the time of pollination:
- Wet Stigma: This type is covered by a sticky secretion, found in plants like Aegle mamlos and Petunia hybrida. The exudate is secreted by the endoplasmic reticulum (ER) and extruded through exocytosis. In some cases, such as Lilium, the stigma is non-secretory, and the exudate on the stigma comes from stigmatic papillae and the stylar canal.
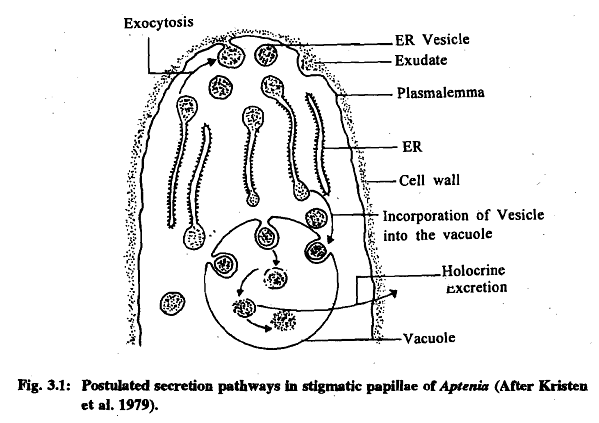
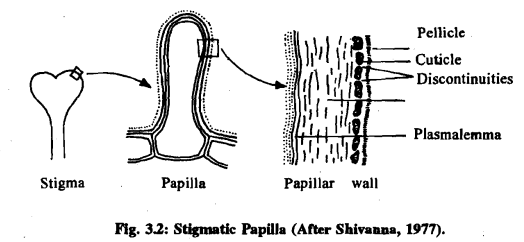
- Dry Stigma: This type lacks any secretion, as seen in cotton. Dry stigmas are covered with long unicellular hairs and have a distinct cuticle at the time of pollination. They also carry lipids and phenolic substances on their surface.
The Style:
The style has two main types:
- Open Style: Found in monocotyledons like Aegle, Fritiliuria, and Lilium, open styles have stylar canals lined with a glandular epidermis. The canal cells in these styles become highly glandular and secretory. In Lilium, each cell may contain 1-5 nuclei, which later fuse. These cells have a large nucleus and organelles such as mitochondria, dictyosomes, and smooth and rough endoplasmic reticulum.
- Closed (Solid) Style: This type, as seen in cotton, consists of an epidermis with stomata, a cortex of thin-walled parenchyma, vascular bundles, and transmitting tissue. The cells of transmitting tissue have thick lateral walls made up of concentric layers. These cells also contain abundant organelles, including mitochondria, amyloplasts, and rough endoplasmic reticulum.
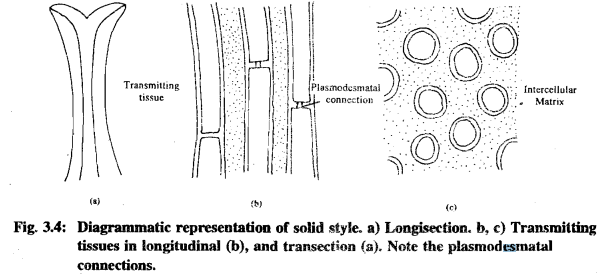
The composition of the stigmatic exudate varies between open and closed styles. Solid style exudates usually contain polysaccharides, lipids, and proteins, while open style exudates primarily consist of polysaccharides.
In wet stigmas like Petunia, the exudate is highly viscous, adhesive, and contains lipids and phenolic compounds. These compounds protect the stigma from desiccation, regulate water availability to pollen, and deter insects. Enzymes from pollen grains on the stigma may release free sugars from phenolic compounds, providing suitable osmotic conditions.
In dry stigmas like cotton, the surface is covered with a cuticle, extracellular proteins, lipids, and phenolic substances. The stigmatic surface carries lateral hairs and a layer of transmitting tissue.
Understanding these interactions and structures is crucial for the successful fertilization of flowering plants.
Pollen Germination-Events on Stigma and in Style
As you've previously learned, the stigma plays a crucial role in providing the right conditions for pollen grains to attach, hydrate, germinate, and develop into pollen tubes (see Fig. 3.5). Stigma receptivity is generally limited to a specific period, occurring before and after anthesis (flowering), and this timing can vary among plant species. The stigma facilitates pollen adhesion, hydration, and germination.
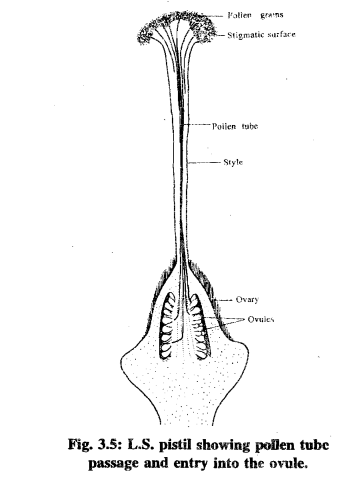
- Adhesion: Pollen grains adhere to the stigma through various mechanisms, including the stickiness of both pollen and stigma surfaces, characteristics of the pollen exine (outer wall), composition of the pellicle (outer coating of pollen), the presence of surface-coat substances, electrostatic forces, and, importantly, specificity between the two parent plants.

- Hydration: Hydration of pollen grains occurs due to the moisture present on the stigmatic surface. On a dry stigma, this hydration process is gradual. Pollen hydration leads to the release of pollen-wall proteins and subsequent interactions between the pollen and the stigma, including compatibility and compatibility/incompatibility responses between the two parent plants.
In plants with a dry stigma and a solid style, an enzyme called cutinase in the pollen tube helps digest the stigmatic cuticle upon contact. The pollen tube then penetrates the pectocellulosic wall of the papillae (stigma cells) and travels through the intercellular spaces of the stigma and style. In contrast, in plants with a dry stigma and a hollow style, the pollen tube grows through the subcuticular mucilage covering the papillae. For plants with a wet stigma and a closed style, the pollen tube enters the intercellular matrix of the stigma before progressing into the style. In plants with a hollow style, the pollen tube initially grows on the surface of the stigma before entering the stylar canal.
- Proteins and Enzymes: Proteins from the tapetum (a layer of cells in the pollen grain wall) play a role in pollen germination, pollen tube penetration, and early pollen tube growth. These proteins also contribute to recognition responses that control inter- and intra-specific compatibility and incompatibility. Gametophytic proteins from the microspore cytoplasm are involved in pollen germination and early nutrition of the pollen grain, as well as in gametophytically controlled incompatibility systems. Sporophytic enzymes from the pollen grain wall are released shortly after pollination, while gametophytic enzymes are slower to move and are detected several minutes later.
In some plant species, like those in the mustard family, the stigmatic papillae are covered entirely by a cuticle layer, and no exudate is produced. Pollen grains enzymatically break down this cuticle, allowing direct contact with the stigmatic papillae. Water is then absorbed by the pollen grains from the turgid (firm and swollen) cells of the stigmatic surface, enabling pollen germination. The stigmatic papillae in these species consist of an outer cuticle layer, a thin intermediate layer of pectin, and an inner layer of pectin and cellulose. Pollen tubes penetrate the cuticle and the intermediate pectic layer, and they grow between the cellulosic lamellae of the innermost pectin-cellulose layer by dissolving only pectic components of the wall. In some cases, such as in B. okracea, an additional waxy layer covers the stigmatic papillae, and pollen grains must pierce this layer before contacting the cuticle. Enzymes responsible for breaking down cutin and pectin have been identified in pollen grains. Understanding these processes is essential for successful fertilization in plants.
The Passage of Pollen Tube
- In cotton plants, the pollen grain quickly forms a tube within an hour of pollination. This tube initially grows on the surface of the stigmatic hairs and then proceeds between the cells of the stigma, particularly at the bases of the hairs. The cytoplasm in the stigmatic hair degenerates during this process, and there is no secretion of exudate. The tube continues its growth by moving through the intercellular spaces within the thin-walled cells of the transmitting tissue. Once it reaches the thick-walled cells of the main strand, it even grows through wall layer 3.
- For plants like Petunia, reports indicate that the pollen tube grows within the compact matrix of the middle lamella of the transmitting tissue. It accomplishes this by enzymatically creating a pipe-like path in front of it. The increased activity of dictyosomes within the cells contributes to the thickening of these cells.
- Callose, a polysaccharide, is deposited in pit fields on the transmitting tissue after the pollen tube's passage. This deposition likely alters cell permeability and is formed as a response to injury and as a reaction to prevent cell leakage.
- The path of the pollen tube through the stigma and style is influenced by the nature and structure of cell walls and the distribution of the transmitting tissue.
Nutrition of Pollen Tubes
The transmitting tissue serves a nutritive role. Pollen tubes of various plants, such as Lilium, Petunia, and Oenothera, draw nourishment, including sugars and amino acids, from the stylar tissue. As the tubes grow through the style, they cause an increased flow of carbohydrates into the pistils. Starch concentration in cells surrounding the stylar canals can change before and after pollination, and this starch is digested as pollen tubes grow.
Metabolism of Pollen Tubes
Pollen grains contain auxins and gibberellins, which play a role in ovary enlargement and fruit development following pollination. Even pollen from unrelated species, nonviable pollen, or pollen extracts can trigger ovary swelling and the formation of seedless fruits. Initially, small amounts of auxins and gibberellins supplied by germinating pollen activate minimal growth and metabolic processes. These substances stimulate the release of additional auxins from the style and ovary tissues, resulting in fruit development.
Respiration of Pollen Tubes
Pollen tubes primarily grow aerobically through the stigmatic and stylar tissues. However, in the lower regions of the style and ovary, they may encounter anaerobic conditions.
Structure of Pollen Tubes
- Pollen tubes in the stigma contain cytoplasm filled with mitochondria and dictyosomes. As the tubes grow, the number of dictyosome cisternae decreases. Large vesicles associated with dictyosomes seem to merge with the pollen tube's wall. Abundant endoplasmic reticulum (ER) and polysomes, either free or attached to ER, can be observed. The cytoplasm also contains various-sized vesicles, ribosomes, and a few underdeveloped plastids.
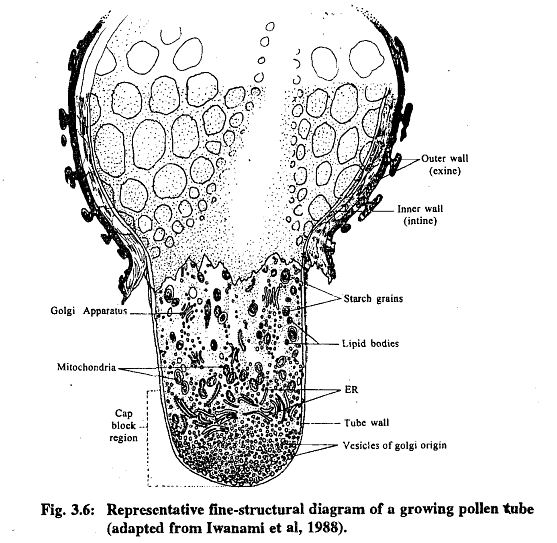
- The pollen tube wall in the stigma and style consists of two distinct regions: an outer wall (PAS positive) and a thicker inner portion, which is less reactive to PAS staining and rich in callose. As the tube matures, its cytoplasm becomes concentrated near the wall, with a large vacuole occupying the remaining space. Wall plugs, mainly composed of callose, separate the older parts of the pollen tube from the growing tip. These plugs originate as rings on the inner wall and grow inward.
Pollen Tube Growth
- In compatible pistils, pollen tubes show deep embayments at their tips but no compartments, while tubes in incompatible pistils have a compartmented cap. Compatible tubes transition from autotrophic nutrition to heterotrophic nutrition as they grow. This transition involves secretion products from stylar canals entering the pollen tubes through the deep embayments. Pollen tubes in incompatible pistils are unable to make this transition, leading to growth cessation due to the depletion of endogenous food reserves.
- The main component of the pollen tube wall is polysaccharides. Since pollen grains have limited food reserves, a significant portion of the material required for pollen tube wall formation likely comes from the polysaccharides present in pistil tissues. In the case of Lilium longiflorum, it has been observed that the polysaccharide component of the stigmatic exudate becomes part of the growing pollen tube's cytoplasm. Later, a specific fraction of this incorporated exudate undergoes extensive metabolism before being used for pollen tube wall biosynthesis.
Pollen Tube Growth in Vitro
In some plant species, it's possible to cultivate pollen tubes in a laboratory setting. However, the maximum length that pollen tubes from species like lilies can achieve in vitro is approximately one centimeter, while the pistil of a lily is ten times longer. This indicates that the pistil provides the necessary conditions for optimal tube growth. In laboratory experiments, a constant nutrient medium is used, although the actual environment for the tube may change as it grows through the pistil.
Several factors have been found to stimulate pollen tube growth in vitro:
- High Relative Humidity: Maintaining a high relative humidity is crucial for pollen germination.
- Carbohydrates: Sugars, particularly sucrose, are essential as they control osmotic pressure and serve as respiratory substrates.
- Boron: Pollen often lack boron, which is compensated for by its presence in the stigma and style. Boron helps reduce bursting of pollen tubes, aids in sugar transport, and affects enzymatic steps involved in carbohydrate biosynthesis.
- Calcium: Calcium ions (Ca++) play a crucial role in pollen tube growth. They promote vigorous, straight, and rigid tube growth and control tube permeability. Calcium's effects are influenced by factors like osmotic conditions, oxygen levels, borate, methyl donors, and other inorganic cations like Mg++, K+, Na+, and H+.
- Enzymes: Enzymes like cellulase, pectinase, and callase are naturally present in pollen grains and can increase the rate of tube elongation when present in the growth medium.
- Plant Hormones: Plant hormones, including auxins and gibberellins, promote tube growth.
- Germination Medium: The growth medium typically contains components like sucrose, H3BO3, Ca(NO3)2, MgSO4, and KNO3. Raffinose-containing media have been known to yield better growth in some cases. Occasionally, minerals like cobalt and zinc have been reported to stimulate pollen tube growth.
- Physical factors, such as temperature (between 20-30°C), also play a role in pollen tube growth.
Fine Structure of Pollen Tubes Grown in Vitro:
- Pollen tubes primarily elongate at their tips, and this tip zone is rich in RNA and protein. Numerous vesicles and a complex network of smooth membranes are present in this zone. Vesicles seem to originate from the ends of dictyosome cisternae, merge with each other, and contribute their contents to the compartmented cap covering the growth zone at the tip.
- Both the cap and vesicles contain pectin, while RNA resides in the smooth membranes. In the region behind the tip, the tube contains organelles present in the pollen before germination, along with numerous amyloplasts. The tube wall is thin, unlike the complex wall observed in tubes growing in pistils. Cellulose is the primary component of the tube wall. Interestingly, the cytoplasm of pollen tubes grown in vitro lacks microtubules.
- The generative cell within the pollen tube is enclosed by its distinct wall, and its cytoplasm differs from that of the pollen grain. This cytoplasm contains only a small number of poorly developed organelles with limited storage material.
In summary, pollen chemistry and growth studies have led to several general conclusions:
- Metabolic pathways in pollen are similar to those in most non-green tissues.
- The composition and balance of chemical constituents in pollen can vary based on species, plant nutrient levels, and developmental environment.
- Certain enzymes or chemical constituents can rapidly diffuse out of pollen.
- Chemicals released from pollen or present on the pollen surface can interact with pistil tissues.
- Tube growth can be influenced by specific chemicals.
- Tube extension occurs through the addition of pectin and hemicellulose via vesiculated membrane-like components at the tip, with cellulose likely added after the initial tube membrane formation.
- Reduced pollen viability after dehiscence is often related to enzyme activities that metabolize endogenous substrates.
Syngamy and Triple Fusion in Angiosperms
In angiosperms, double fertilization is a crucial reproductive process involving the fusion of two male gametes with different female nuclei. This process consists of syngamy, where one male gamete fuses with the egg to form the zygote, and triple fusion, where the other male gamete fuses with the fusion product of two polar nuclei, resulting in the formation of the primary endosperm nucleus (which has a triploid chromosome number, denoted as 3n).
Entry of Pollen Tube into the Embryo Sac
The pollen tube's ultimate goal is to reach the female gametophyte and release the male gametes to ensure fertilization. Typically, the pollen tube enters the embryo sac through the filiform apparatus of one of the synergids. Before the entry of the pollen tube, one of the synergids often degenerates, and the tube usually enters through this degenerating synergid. However, in some cases, both synergids remain healthy until the pollen tube's entry, and the one that receives the pollen tube starts to degenerate. This degeneration process may be essential to prevent rejection or an antigen-antibody-type reaction between the male gametes and the female reproductive structures.
Choice of Male Gametes
The choice of which male gamete is involved in syngamy and which one participates in triple fusion has been a subject of interest among embryologists. Studies using transmission electron microscopy and scanning electron microscopy have shown that in certain plants, the two male gametes differ in size, plastid content, and mitochondria. It appears that the sperm with more plastids tends to participate in syngamy, while the one with fewer plastids fuses with the secondary nucleus. However, recent research suggests that not all plants exhibit this heteromorphic condition with unequal sperm.
Syngamy
- The pollen tube's growth within the synergid is limited, and it releases its contents, including the two male gametes, along with cytoplasm, reserve nutrients, and possibly the vegetative nucleus, through a terminal or subterminal pore. One of the sperms enters the egg cell, and the other enters the central cell. The distance the male gamete has to travel to reach either the egg or secondary nucleus is quite short.
- When the male gamete comes into contact with the plasma membrane of the egg, a bridge forms through which the male gamete enters. Nuclear fusion begins as the outer membranes of the two nuclei join. The inner membranes also fuse at specific areas, forming a bridge between the two nuclei. The expansion of this bridge eventually leads to the fusion of the male and egg nuclei, resulting in syngamy. Three types of syngamy have been identified based on the timing of nuclear fusion: pre-mitotic, post-mitotic, and intermediate. Generally, the male cytoplasm does not actively participate in fertilization, although there are exceptions in plants where both parents contribute to plastid inheritance.
Triple Fusion
The fusion process between the other male gamete and the secondary nucleus follows a similar pattern as syngamy. In most plants, the polar nuclei are only partially fused when the male gamete approaches. The fusion of the male gamete with one of the polar nuclei completes the fusion process of all three nuclei (triple fusion). Interestingly, syngamy begins before triple fusion but triple fusion is completed first.
|
179 videos|143 docs
|





















