DNA Sequencing | Botany Optional for UPSC PDF Download
| Table of contents |

|
| Introduction |

|
| Decoding the Genetic Blueprint |

|
| Sanger Sequencing: The Chain Termination Method |

|
| Ingredients for Sanger sequencing |

|
| Method of Sanger sequencing |

|
| Next-generation sequencing |

|
Introduction
In the world of biotechnology, there exists a groundbreaking process known as DNA sequencing, a pivotal technique that has forever changed our understanding of genetics and genomics. This article delves into the intricacies of DNA sequencing, exploring its history, methodologies, and the remarkable advancements that have shaped the field. From the classic Sanger sequencing to the futuristic realm of next-generation sequencing, readers will gain insight into the evolution of this vital biotechnological tool.
Decoding the Genetic Blueprint
- At the heart of DNA sequencing lies the endeavor to decipher the sequence of nucleotide bases—Adenine (A), Thymine (T), Cytosine (C), and Guanine (G)—that constitute the DNA molecule. DNA sequencing is essentially the process of revealing the precise order of these bases within a DNA fragment or an entire genome. While the sequencing of a short DNA segment may seem relatively straightforward today, sequencing an entire genome is a complex task that involves fragmenting the DNA, sequencing those fragments, and assembling them into a comprehensive genome.
- The monumental Human Genome Project, completed in 2003 through international collaboration, marked a significant milestone in genomics. It employed Sanger sequencing to determine the sequences of numerous smaller fragments of human DNA. These fragments were later aligned based on overlapping portions, allowing scientists to assemble the sequences of larger DNA regions and entire chromosomes. Over the past two decades, advancements in sequencing techniques have drastically reduced the time and cost associated with genome sequencing, making it more accessible than ever before.
Sanger Sequencing: The Chain Termination Method
- Sanger sequencing, also known as the chain termination method, has been a cornerstone of DNA sequencing for decades. Developed by British biochemist Fred Sanger and his colleagues in 1977, this method remains instrumental in sequencing DNA fragments of up to approximately 900 base pairs.
- During Sanger sequencing, a key component is the utilization of dideoxy, or chain-terminating, versions of nucleotides (ddATP, ddTTP, ddCTP, ddGTP), each labeled with distinct dye colors. These dideoxy nucleotides lack a hydroxyl group on the 3' carbon of the sugar ring, preventing further nucleotide addition after their incorporation. As a result, the DNA strands terminate with dideoxy nucleotides, each marked with a specific dye color corresponding to its base (A, T, C, or G).
- The Sanger sequencing process commences with the combination of the target DNA, a primer, DNA polymerase, regular DNA nucleotides (dATP, dTTP, dGTP, and dCTP), and the dideoxy nucleotides in small quantities. Cycling through denaturation, primer binding, and DNA synthesis stages, the reaction produces DNA fragments of varying lengths, each concluding with a dideoxy nucleotide.
- To determine the sequence, these fragments are subjected to capillary gel electrophoresis. Short fragments move swiftly through the gel pores, while longer ones move more slowly. As each fragment crosses the finish line, illuminated by a laser, its attached dye is detected, yielding a series of peaks in fluorescence intensity. By analyzing these peaks, scientists can meticulously reconstruct the original DNA sequence, base by base.
Ingredients for Sanger sequencing
Sanger sequencing involves the amplification of a target DNA region and requires similar components to those used in DNA replication within an organism or in the polymerase chain reaction (PCR) for in vitro DNA copying.
These components include:
- DNA polymerase enzyme
- A primer, a short single-stranded DNA fragment that binds to the template DNA, initiating polymerase activity.
- The four DNA nucleotides (dATP, dTTP, dCTP, dGTP)
- The template DNA segment to be sequenced
However, Sanger sequencing distinguishes itself by incorporating a unique component:
Dideoxy (chain-terminating) versions of all four nucleotides (ddATP, ddTTP, ddCTP, ddGTP), each tagged with a distinct color of dye.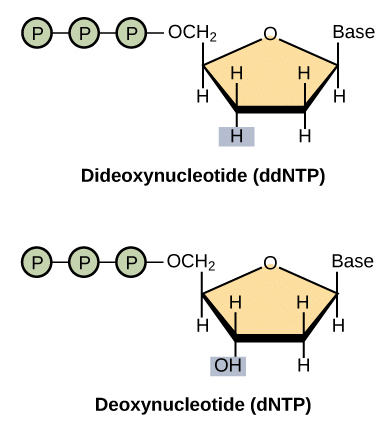
Dideoxy nucleotides are similar to regular (deoxy) nucleotides but lack a critical feature: the hydroxyl group on the 3' carbon of the sugar ring. In regular nucleotides, this 3' hydroxyl group serves as an attachment point, enabling the addition of new nucleotides to an existing DNA chain. However, once a dideoxy nucleotide is incorporated into the chain, it lacks the necessary 3' hydroxyl group, preventing further nucleotide addition. This results in the termination of the chain, and the specific dye color indicates which base (A, T, C, or G) is present in the terminated position.
Method of Sanger sequencing
The Sanger sequencing method involves several steps:
- Combining the DNA sample to be sequenced with a primer, DNA polymerase, regular DNA nucleotides (dATP, dTTP, dGTP, and dCTP), and small quantities of dye-labeled, chain-terminating dideoxy nucleotides.
- Heating the mixture to denature the template DNA, separating its two strands.
- Cooling the mixture to enable the binding of a primer to the single-stranded template.
- Raising the temperature again, allowing DNA polymerase to initiate the synthesis of new DNA strands from the primer. DNA polymerase will continue to add nucleotides to the growing chain until, by chance, it incorporates a dideoxy nucleotide instead of a regular one. When this happens, further nucleotide addition is halted, and the strand ends with the dideoxy nucleotide.
- Repeating this process through several cycles. By the end of these cycles, it is highly likely that a dideoxy nucleotide has been inserted at every position along the target DNA sequence in at least one reaction. Consequently, the reaction tube will contain DNA fragments of varying lengths, each terminating at one of the original nucleotide positions. These fragments will be labeled with dyes indicating their respective final nucleotides.
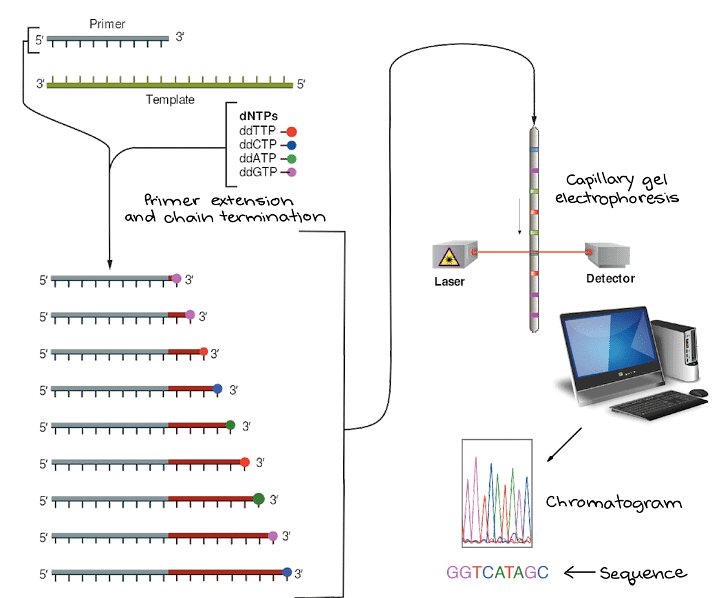
Uses and Limitations
- Sanger sequencing provides accurate DNA sequences for relatively lengthy DNA fragments, reaching up to approximately 900 base pairs. This method is commonly employed for sequencing isolated DNA segments, including bacterial plasmids or DNA generated through PCR.
- Nonetheless, Sanger sequencing proves costly and inefficient when applied to extensive projects, such as sequencing entire genomes or metagenomes (the combined genetic material of a microbial community). For these types of tasks, more recent large-scale sequencing methods offer quicker and more cost-effective solutions.
Next-generation sequencing
Next-generation sequencing (NGS), despite its sci-fi sounding name, is the collective term for the latest advancements in DNA sequencing technologies.
Various NGS techniques employ different technologies, but they share common characteristics that set them apart from Sanger sequencing:
- Highly Parallel: Multiple sequencing reactions occur simultaneously.
- Micro Scale: Reactions are conducted on a small scale, allowing many to be performed simultaneously on a single chip.
- Rapid: Due to parallel processing, results are available much more quickly.
- Cost-Effective: Sequencing entire genomes is more economical compared to Sanger sequencing.
- Shorter Reads: Typically, the sequences generated are shorter, ranging from 50 to 700 nucleotides.
In essence, NGS can be thought of as conducting numerous miniature Sanger sequencing reactions concurrently. Thanks to this parallelization and reduced scale, NGS methods enable the swift and cost-efficient sequencing of large quantities of DNA when compared to traditional Sanger sequencing. To illustrate, the cost of sequencing a human genome dropped significantly from nearly $100 million in 2001 to just $1245 in 2015.
|
179 videos|143 docs
|



















