Photosynthesis: Photochemical reactions, Photophosphorylation & Carbon fixation pathways | Botany Optional for UPSC PDF Download
Photochemical Reaction
Photochemistry, the captivating realm of chemistry, delves into the mesmerizing chemical transformations sparked by the absorption of radiant light energy. Within this captivating domain, a photochemical reaction emerges as a chemical metamorphosis initiated by the infusion of energy in the form of photons, radiating distinctive products. In essence, this article unveils the intricate facets of photochemical reactions, their historical underpinnings, significance, foundational principles, categorizations, and practical applications.
Understanding Photochemical Reactions: A Molecular Dance with Light
- Photochemical reactions represent a captivating phenomenon wherein molecules, typically residing in their lowest energy state, known as the ground state, undergo a remarkable transformation when excited by photons. This luminous excitement propels them into a transient, enigmatic state known as the excited state, wherein their physical and chemical attributes differ significantly from those in the ground state.
- These reactions hinge on the critical role played by photons in activating molecules, thus instigating a chain reaction that can engender new molecular structures or transfer energy to other molecules. Crucially, this intricate dance between molecules and light can transpire within solids, liquids, and gases.
- The roots of photochemistry trace back to the early 1800s, with pivotal contributions by visionaries like German physicist Theodor von Grotthus and American chemist John William Draper. Their endeavors culminated in a profound comprehension of photochemical processes, underpinning the foundation of modern photochemistry.
The Pivotal Role of Photochemical Reactions in Our World
- The reverberations of photochemical reactions extend far beyond the confines of the laboratory. They are integral to the sustenance of life on our beloved Earth, instigating chemical alterations within the atmospheric gases under the influence of solar radiation and suspended particles.
- Through the lens of photochemistry, we gain insights into crucial global phenomena such as ozone layer depletion, the specter of acid rain, and the haunting shadow of global warming. What sets photochemical reactions apart is their dependency on sunlight, an abundant and omnipresent energy source, which may have also played a role in the inception of life itself on our primordial planet.
- Intriguingly, photochemical processes epitomize perfect atom economy, as the transformation commences with a photon, obviating the need for additional reagents.
Demystifying the Fundamental Principles of Photochemical Reactions
At the heart of photochemical reactions lie two immutable laws of photochemistry:
- Grothuss-Draper Law: This cardinal decree asserts that a molecule must engage with light through absorption for a chemical reaction to manifest.
- Stark-Einstein Law: This fundamental principle posits that each photon absorbed by a molecule activates precisely one molecule for ensuing reactions. The efficiency of these reactions finds expression in the concept of quantum yield, denoting "the number of moles of a stated reactant disappearing, or the number of moles of a stated product produced, per mole of a photon of monochromatic light absorbed." The quantum yield serves as a yardstick for the efficacy of specific photochemical processes.
Diverse Avatars of Photochemical Reactions: A Taxonomic Odyssey
Photochemical reactions unveil a captivating spectrum of transformations, including:
- Photo-dissociation: Where molecules split into fragments upon photon absorption.
- Photo-induced rearrangements and isomerization: In which molecules undergo structural shifts upon exposure to light.
- Photo-addition: Occurring when molecules combine, driven by the infusion of photons.
- Photo-substitution: Where molecules exchange constituents in response to photon bombardment.
- Photo-redox reactions: Marked by the transfer of electrons or the alteration of oxidation states in molecules under the influence of photons.
Illuminating Instances: A Glimpse into the World of Photochemical Reactions
Photochemical reactions manifest in diverse facets of life:
- Photosynthesis: Plants harness the energy of sunlight to convert carbon dioxide and water into glucose and oxygen, fueling life on our planet.
- Photography: The magic of capturing images is made possible through the decomposition of silver halides like AgCl and AgBr upon exposure to light.
- Solar Cells: These marvels of technology convert solar energy into electrical energy, propelling space exploration.
- Vitamin D Synthesis: Our skin synthesizes vitamin D when exposed to sunlight.
- Carbonyl Compounds: These undergo various photochemical reactions in gaseous and liquid phases, contributing to diverse chemical processes.
The Atmospheric Theater: Photochemical Reactions at Play
Our atmosphere plays host to a dynamic theater of photochemical reactions. Under the ceaseless bombardment of solar radiation, atmospheric molecules absorb light energy, inducing intricate chemical transformations. These reactions are the architects of the chemical composition of our atmosphere, influencing pollutant species and beyond.
The Enigma of Photochemical Smog: Unveiling the Reactions
- Photochemical smog, a complex assemblage of pollutants, materializes when nitrogen oxides (NOx) and volatile organic compounds (VOCs) engage in a dance with sunlight, yielding a brown haze that shrouds urban landscapes. This byproduct of industrialization exerts a far-reaching impact, not only on the environment but also on human health and materials.
- Key actors in this smog symphony include nitrogen oxides, ozone, and peroxyacetyl nitrate (PAN), with nitrogen oxides and VOCs serving as primary players, while ozone, aldehydes, and PAN assume secondary roles. While ozone in the stratosphere shields us from harmful ultraviolet rays, ground-level ozone poses health hazards.
- The reactions orchestrating photochemical smog formation encompass:
- The absorption of ultraviolet light by nitrogen dioxide (NO2), leading to the creation of nitric oxide (NO) and atomic oxygen (O).
- The genesis of ozone (O3) through the interaction of molecular oxygen (O2) with atomic oxygen.
- The reaction of ozone with nitric oxide (NO), yielding nitrogen dioxide (NO2) and molecular oxygen.
- The formation of peroxyacetyl nitrate (PAN) through the union of nitrogen dioxide with diverse hydrocarbons (RH) emanating from VOCs.
- The conversion of oxygenated organic and inorganic compounds (ROx) into more nitrogen oxides through their interaction with nitric oxide.
Harnessing the Radiance: Industrial Applications of Photochemical Reactions
In the world of industry, photochemical reactions serve as invaluable tools for:
- Crafting the anti-malarial drug.
- Producing benzyl chloride.
- Synthesizing a multitude of synthetic organic molecules.
Deciphering the Dichotomy: Photochemical Reaction vs. Thermal Reaction
The juxtaposition between photochemical reactions and their thermal counterparts unveils notable distinctions:
Discerning the Divide: Photochemical Reaction vs. Electrochemical Reaction
A distinctive chasm separates photochemical reactions from electrochemical reactions:

Photophosphorylation
In the realm of cellular energy production, one fascinating process stands apart - Photophosphorylation. This unique mechanism is exclusive to cells engaged in photosynthesis, distinguishing itself from the more familiar oxidative phosphorylation. While both share certain similarities, such as utilizing a membrane-associated electron transport chain and harnessing a proton gradient to generate ATP with the assistance of ATP synthase, the fundamental distinction lies in their energy sources.
The Essence of Photophosphorylation: Solar Power
Unlike oxidative phosphorylation, where ATP synthesis derives energy from the oxidation of biological molecules and the subsequent production of electrons, photophosphorylation taps into the sun's radiant energy. In the intricate dance of photosynthesis, photons from the sun encounter chlorophyll molecules within reaction centers located in chloroplasts (refer to Figures 5.3.1 and 5.3.2). This pivotal interaction serves as the catalyst for photophosphorylation, setting it apart from its oxidative counterpart in several key aspects:
- Source of Electrons: Photosynthesis relies on water (H2O) as the source of electrons, while oxidative phosphorylation primarily draws its electrons from NADH/FADH2.
- Direction of Proton Pumping: In photosynthesis, protons are pumped into the thylakoid space within chloroplasts, while in oxidative phosphorylation, protons move out of the mitochondrial matrix.
- Proton Movement during ATP Synthesis: During ATP synthesis in photosynthesis, protons flow out of the thylakoid space, whereas in oxidative phosphorylation, they enter the mitochondrial matrix.
- Terminal Electron Acceptor: Photosynthesis employs NADP+ as the terminal electron acceptor, whereas oxidative phosphorylation utilizes oxygen (O2).

Electron Transport: Chloroplasts vs. Mitochondria
Intriguingly, the movement of electrons within chloroplasts during photosynthesis operates in reverse compared to electron transport in mitochondria. In the photosynthetic realm, water donates electrons, with their ultimate destination being NADP+ to form NADPH. In contrast, mitochondria source electrons from NADH/FADH2, and water serves as their final resting place. The key to making electrons traverse this dual path lies in the energy harnessed from solar photons, elevating electrons to an energy level that propels them toward their NADPH destination in a Z-shaped sequence.
It's important to note that photosynthesis encompasses two distinct phases: the light cycle, as described above, and the dark cycle, known as the Calvin Cycle, responsible for capturing atmospheric CO2 and converting it into glucose.

Photosynthesis: Nature's Energy Capture
Photosynthesis is a vital energy capture process deployed by plants and various organisms to harness light energy, transforming it into chemical energy. This chemical energy is ultimately stored in carbohydrates, synthesized through the utilization of ATP (derived from energy harvesting), carbon dioxide, and water. Additionally, photosynthesis often yields oxygen as a byproduct, released during the water capture process. This process not only contributes to the Earth's atmospheric oxygen but also serves as the primary source of organic materials and energy for life on our planet.
The Steps of Photosynthesis
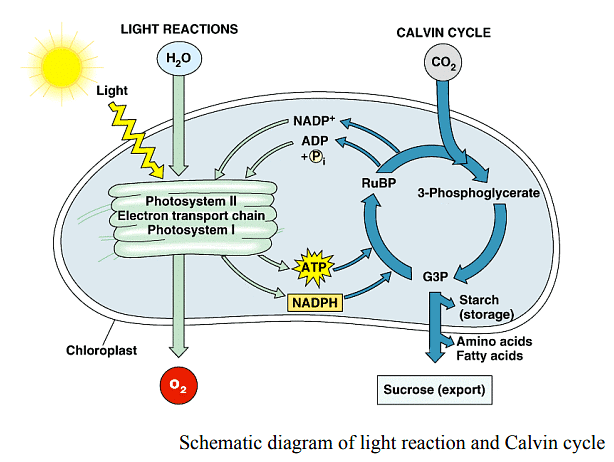
While the precise steps of photosynthesis vary slightly among different organisms, the process begins with the capture of energy from light by protein complexes containing chlorophyll pigments, referred to as reaction centers. In plants, these proteins reside within chloroplasts, while bacteria, devoid of organelles, embed them in their plasma membranes. Light energy is utilized to strip electrons from electron donors, typically water, releasing oxygen as a byproduct. Electrons are then donated to a carrier and eventually accepted by NADP+, forming NADPH. The movement of electrons generates a proton gradient across the thylakoid membrane, facilitating ATP synthesis. Consequently, the initial phase of photosynthesis, termed the light reactions, yields NADPH, ATP, and oxygen.
The Intricacies of Chloroplasts
Chloroplasts are ubiquitous in aboveground plant cells, with a concentration in leaves. Within a leaf, the photosynthetic tissue, called mesophyll, can house up to a staggering 800,000 chloroplasts per square millimeter. The chloroplast's structure comprises a phospholipid inner membrane, a phospholipid outer membrane, and an intermembrane space. The stroma, found within the inner chloroplast membrane, houses chloroplast DNA and the enzymes involved in the Calvin Cycle. Stacked, flattened disks known as thylakoids, each defined by thylakoid membranes, are also found within the stroma. Thylakoid spaces or thylakoid lumen refer to the spaces within these membranes.

Illuminating the Light Reactions
- Within chloroplasts, the light reactions of photosynthesis, centered around electron transfer, occur in the thylakoid membranes. On the other hand, the assimilation of carbon dioxide to produce glucose involves separate biochemical reactions known as the Calvin cycle or "dark reactions," which will be covered in detail elsewhere.
- The magic of the thylakoid membrane unfolds with the participation of four major protein complexes: Photosystem II (PS II), Cytochrome b6f complex (Cb6f), Photosystem I (PS I), and ATP synthase. These complexes play distinct roles, such as capturing light energy, creating a proton gradient via electron movement, capturing light energy once again, and using the proton gradient's energy to synthesize ATP.

The Dance of Electrons and Protons
Harvesting the energy of light initiates in Photosystem II (PS II), where a photon's absorption triggers an electron's excitation. This electron, readily relinquished by PS II, requires replacement. The Oxygen Evolving Complex (OEC) steps in as an intermediate, containing four manganese centers that provide the immediate electron replenishment PS II demands. After OEC supplies four electrons to PS II, it extracts four electrons from two water molecules, liberating oxygen and contributing to the proton gradient by releasing four protons into the thylakoid space. The excited electron from PS II swiftly passes its charge to pheophytin, which, in turn, transfers the electron to protein-bound plastoquinones. Plastoquinone A (PQA) ushers the electron to Plastoquinone B (PQB), which waits for a second electron while capturing two protons, transforming into PQH2, also known as plastoquinol. PQH2 then shuttles these components to the Cytochrome b6f complex (Cb6f), which pumps protons into the thylakoid space. ATP synthase capitalizes on this proton gradient to generate ATP. Cb6f subsequently delivers the electron to plastocyanin, retaining it until the next excitation cycle commences with the absorption of another photon by Photosystem I (PS I).
The Power of Photosynthetic Energy
- The output of the photophosphorylation process, which includes oxygen (O2), NADPH, and ATP, is merely the beginning of photosynthesis's intricate journey. For plants and other growing organisms, NADPH and ATP serve as tools to capture carbon dioxide from the atmosphere, ultimately transforming it into glucose and essential carbon compounds. This essential step occurs in the Calvin Cycle, commonly referred to as the dark phase of photosynthesis. The oxygen liberated during this process is indispensable for the respiration of all aerobic life forms on Earth, with a substantial portion of the planet's atmospheric oxygen attributed to the eons of water splitting in photosynthesis.
- In essence, photophosphorylation is a captivating example of nature's ability to harness solar power and convert it into the vital energy stores that sustain life on our planet. Its intricate dance of electrons, protons, and photons highlights the elegance of photosynthesis, a process that has shaped our world for countless millennia.
Dark reaction or Blackman’s reaction or Path of carbon in photosynthesis
- The dark reaction, also known as Blackman's reaction or the carbon pathway in photosynthesis, represents the second stage of the photosynthesis process. It's a set of chemical processes that occur independently of light and takes place within the stroma of chloroplasts. Dark reactions are primarily enzyme-driven and operate at a slower pace compared to the light-dependent reactions. However, it's important to note that dark reactions can still occur in the presence of light.
- During the dark reaction, carbon dioxide (CO2) is transformed into sugars. This transformation involves the conversion of the energy-poor CO2 into energy-rich carbohydrates, utilizing the energy-rich molecules ATP and the assimilatory power NADPH2 produced during the light-dependent reactions. This process is referred to as carbon fixation or carbon assimilation. The name "Blackman's reaction" is used because it was Blackman who initially demonstrated the existence of these dark reactions.
Calvin cycle or C3 cycle
There are two main types of cyclic reactions that occur within the dark reaction:
- Calvin Cycle or C3 Cycle: This cyclic process operates during the dark phase of photosynthesis and is responsible for converting CO2 into sugars, thus fixing carbon. Melvin Calvin first observed the Calvin cycle in unicellular green algae called chlorella, and he was awarded the Nobel Prize for this discovery in 1961. The term "C3 cycle" is derived from the fact that the first stable compound in this cycle is a three-carbon molecule called 3-phosphoglyceric acid.
The Calvin cycle can be divided into three phases:- Carboxylative phase
- Reductive phase
- Regenerative phase

1. Carboxylative phase
Three molecules of CO2 are accepted by 3 molecules of 5C compound viz., ribulose diphosphate to form three molecules of an unstable intermediate 6C compound. This reaction is catalyzed by the enzyme, carboxy dismutase The three molecules of the unstable 6 carbon compound are converted by the addition of 3 molecules of water into six molecules of 3 phosphoglyceric acid. This reaction is also catalyzed by the enzyme carboxy mutase.
The three molecules of the unstable 6 carbon compound are converted by the addition of 3 molecules of water into six molecules of 3 phosphoglyceric acid. This reaction is also catalyzed by the enzyme carboxy mutase. 3 phosphoglyceric acid (PGA) is the first stable product of dark reaction of photosynthesis and since it is a 3 carbon compound, this cycle is known as C3 cycle.
3 phosphoglyceric acid (PGA) is the first stable product of dark reaction of photosynthesis and since it is a 3 carbon compound, this cycle is known as C3 cycle.
2. Reductive phase
Six molecules of 3PGA are phosphorylated by 6 molecules of ATP (produced in the light reaction) to yield 6 molecules of 1-3 diphospho glyceric acid and 6 molecules of ADP. This reaction is catalyzed by the enzyme, Kinase Six molecules of 1, 3 diphosphoglyceric acid are reduced with the use of 6 molecules of NADPH2 (produced in light reaction) to form 6 molecules of 3 phospho glyceraldehyde. This reaction is catalysed by the enzyme, triose phosphate dehydrogenase.
Six molecules of 1, 3 diphosphoglyceric acid are reduced with the use of 6 molecules of NADPH2 (produced in light reaction) to form 6 molecules of 3 phospho glyceraldehyde. This reaction is catalysed by the enzyme, triose phosphate dehydrogenase.
3. Regenerative phase
In the regenerative phase, the ribose diphosphate is regenerated. The regenerative phase is called as pentose phosphate pathway or hexose monophophate shunt.
It involves the following steps:
- Some of the molecules of 3 phospho glyceraldehyde into dihydroxy acetone phosphate. Both 3 phospho glyceraldehyde and dihydroxy acetone phosphate then unite in the presence of the enzyme, aldolase to form fructose, 1-6 diphosphate.

- Fructose 6 phosphate is converted into fructose 6 phosphate in the presence of phosphorylase

- Some of the molecules of 3 phospho glyceraldehyde instead of forming hexose sugars are diverted to regenerate ribulose 1-5 diphosphate

- 3 phospho glyceraldehyde reacts with fructose 6 phosphate in the presence of enzyme transketolase to form erythrose 4 phosphate ( 4C sugar) and xylulose 5 phosphate(5C sugar)

- Erythrose 4 phosphate combines with dihydroxy acetone phosphate in the presence of the enzyme aldolase to form sedoheptulose 1,7 diphosphate(7C sugar)

- Sedoheptulose 1, 7 diphosphate loses one phosphate group in the presence of the enzyme phosphatase to form sedoheptulose 7 phosphate.

- Sedoheptulose phosphate reacts with 3 phospho glyceraldehyde in the presence of transketolase to form xylulose 5 phosphate and ribose 5 phosphate ( both % c sugars)

- Ribose 5 phosphate is converted into ribulose 1, 5 diphosphate in the presence of enzyme, phosphopentose kinase and ATP. Two molecules of xylulose phosphate are also converted into one molecule of ribulose monophosphate. The ribulose monophosphate is phosphorylated by ATP to form ribulose diphosphate and ADP, thus completing Calvin cycle.

- In the dark reaction, CO2 is fixed to carbohydrates and the CO2 acceptor ribulose diphosphate is regenerated. In Calvin cycle, 12 NADPH2 and 18 ATPs are required to fix 6 CO2 molecules into one hexose sugar molecule (fructose 6 phosphate).

C4 cycle or Hatch and Slack pathway
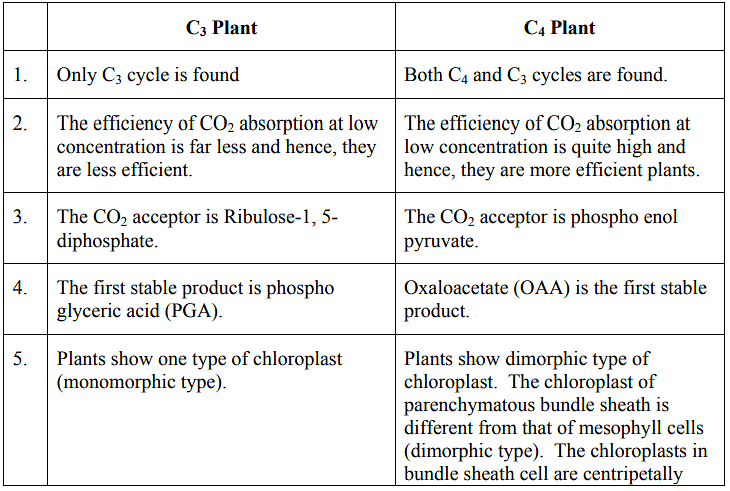
- The C4 cycle, also known as the Hatch and Slack pathway or the C4 dicarboxylic acid pathway, represents an alternative route to fix carbon dioxide (CO2) compared to the C3 cycle in photosynthesis. In this pathway, the first stable compound formed is a four-carbon molecule known as oxaloacetic acid, which is why it's referred to as the C4 cycle. This pathway was elucidated by Hatch and Slack in 1966, and it's commonly found in several plants such as grasses, sugar cane, maize, sorghum, and amaranthus.
- C4 plants exhibit a distinct leaf anatomy. Their chloroplasts come in two different forms. Within the leaves of these plants, the vascular bundles are encircled by a bundle sheath consisting of larger parenchymatous cells. These bundle sheath cells contain chloroplasts. Notably, these bundle sheath chloroplasts are larger, lack grana (stacked thylakoid membranes), and contain starch granules. In contrast, the chloroplasts in mesophyll cells, which are found between the bundle sheath cells, are smaller and always have grana. This unique leaf anatomy of C4 plants is referred to as Kranz anatomy. The bundle sheath cells are larger and resemble a ring or wreath, which is why it's called Kranz anatomy.
- The C4 cycle involves two distinct carboxylation reactions, one occurring in the chloroplasts of mesophyll cells and the other in the chloroplasts of bundle sheath cells.
The Hatch and Slack cycle consists of four main steps:- Carboxylation
- Breakdown
- Splitting
- Phosphorylation
- In summary, the C4 cycle is an alternative pathway for carbon fixation in photosynthesis, characterized by the formation of a four-carbon compound. This pathway was named after the scientists Hatch and Slack, and it is commonly found in certain plants with Kranz anatomy. The C4 cycle involves specific carboxylation reactions in mesophyll and bundle sheath chloroplasts, leading to the production of organic compounds.
1. Carboxylation
It takes place in the chloroplasts of mesophyll cells. Phosphoenolpyruvate, a 3 carbon compound picks up CO2 and changes into 4 carbon oxaloacetate in the presence of water. This reaction is catalysed by the enzyme, phosphoenol pyruvate carboxylase.
2. Breakdown
Oxaloacetate breaks down readily into 4 carbon malate and aspartate in the presence of the enzyme, transaminase and malate dehydrogenase. These compounds diffuse from the mesophyll cells into sheath cells.
3. Splitting
In the sheath cells, malate and aspartate split enzymatically to yield free CO2 and 3 carbon pyruvate. The CO2 is used in Calvin’s cycle in the sheath cell. The second Carboxylation occurs in the chloroplast of bundle sheath cells. The CO2 is accepted by 5 carbon compound ribulose diphosphate in the presence of the enzyme, carboxy dismutase and ultimately yields 3 phosphoglyceric acid. Some of the 3 phosphoglyceric acid is utilized in the formation of sugars and the rest regenerate ribulose diphosphate.
The second Carboxylation occurs in the chloroplast of bundle sheath cells. The CO2 is accepted by 5 carbon compound ribulose diphosphate in the presence of the enzyme, carboxy dismutase and ultimately yields 3 phosphoglyceric acid. Some of the 3 phosphoglyceric acid is utilized in the formation of sugars and the rest regenerate ribulose diphosphate.
4. Phosphorylation
The pyruvate molecule is transferred to chloroplasts of mesophyll cells where, it is phosphorylated to regenerate phosphoenol pyruvate in the presence of ATP. This reaction is catalysed by pyruvate phosphokinase and the phophoenol pyruvate is regenerated. In Hatch and Slack pathway, the C3 and C4 cycles of carboxylation are linked and this is due to the Kranz anatomy of the leaves. The C4 plants are more efficient in photosynthesis than the C3 plants. The enzyme, phosphoenol pyruvate carboxylase of the C4 cycle is found to have more affinity for CO2 than the ribulose diphosphate carboxylase of the C3 cycle in fixing the molecular CO2 in organic compound during Carboxylation.
In Hatch and Slack pathway, the C3 and C4 cycles of carboxylation are linked and this is due to the Kranz anatomy of the leaves. The C4 plants are more efficient in photosynthesis than the C3 plants. The enzyme, phosphoenol pyruvate carboxylase of the C4 cycle is found to have more affinity for CO2 than the ribulose diphosphate carboxylase of the C3 cycle in fixing the molecular CO2 in organic compound during Carboxylation.

Crassulacean Acid Metabolism (CAM) cycle or the dark fixation of CO2 in succulent
- Crassulacean Acid Metabolism (CAM) cycle, also known as the dark fixation of CO2 in succulents, is a cyclic process that occurs during the dark phase of photosynthesis in plants belonging to the Crassulaceae family. It represents a unique way of fixing carbon dioxide (CO2), with malic acid being the initial product. CAM is the third alternative pathway to the Calvin cycle and takes place in the mesophyll cells of these plants. Plants that employ the CAM cycle are referred to as CAM plants, and examples include Bryophyllum, Kalanchoe, Crassula, Sedum, Kleinia, as well as certain species of Cacti, Orchids, and Pineapples.
- CAM plants are typically succulents that thrive in extremely dry conditions. Their leaves are often fleshy or succulent, and the mesophyll cells contain a higher number of chloroplasts. Unlike some other plants, CAM plants do not have well-defined bundle sheath cells surrounding the vascular bundles. These plants have a unique stomatal behavior; they keep their stomata open at night and close them during the daytime. This adaptation allows CAM plants to carry out photosynthesis and survive in arid environments. While CAM plants are not as efficient as C4 plants in photosynthesis, they are better suited to endure extreme dry spells.
- The CAM cycle involves two main steps:
- Acidification: During this step, CO2 is taken up and converted into organic acids, primarily malic acid.
- Deacidification: In this phase, the stored organic acids are broken down, releasing CO2 for use in the subsequent light-dependent reactions of photosynthesis.
- In summary, the CAM cycle is a specialized carbon fixation pathway found in certain plants, particularly succulents, that enables them to adapt to arid conditions by performing photosynthesis primarily during the night when water loss through transpiration is minimized. This adaptation allows them to thrive in environments with extreme desiccation, although they are not as efficient as C4 plants in photosynthesis.
1. Acidification
In darkness, the stored carbohydrates are converted into phophoenol pyruvic acid by the process of Glycolysis. The stomata in CAM plants are open in dark and they allow free diffusion of CO2 from the atmosphere into the leaf. Now, the phosphoenolpyruvic acid carboxylated by the enzyme phosphoenol pyruvic acid carboxylase and is converted in to oxalaoacetic acid. The oxaloacetic acid is then reduced to malic acid in the presence of the enzyme malic dehydrogenase. The reaction requires NADPH2 produced in Glycolysis.
The oxaloacetic acid is then reduced to malic acid in the presence of the enzyme malic dehydrogenase. The reaction requires NADPH2 produced in Glycolysis. The malic acid produced in dark is stored in the vacuole. The malic acid increases the acidity of the tissues.
The malic acid produced in dark is stored in the vacuole. The malic acid increases the acidity of the tissues.
2. Deacidification
During day time, when the stomata are closed, the malic acid is decarboxylated to produce pyruvic acid and evolve carbon dioxide in the presence of the malic enzyme. When the malic acid is removed, the acidity decreases the cells. This is called deacidification. One molecule of NADP+ is reduced in this reaction.  T he pyruvic acid may be oxidized to CO2 by the pathway of Kreb’s cycle or it may be reconverted to phosphoenol pyruvic acid and synthesize sugar by C3 cycle. The CO2 released by deacidification of malic acid is accepted by ribulose diphosphate and is fixed to carbohydrate by C3 cycle.
T he pyruvic acid may be oxidized to CO2 by the pathway of Kreb’s cycle or it may be reconverted to phosphoenol pyruvic acid and synthesize sugar by C3 cycle. The CO2 released by deacidification of malic acid is accepted by ribulose diphosphate and is fixed to carbohydrate by C3 cycle.
CAM is a most significant pathway in succulent plants. The stomata are closed during day time to avoid transpiration loss of water. As the stomata are closed, CO2 cannot enter into the leaves from the atmosphere. However, they can carry out photosynthesis during the day time with the help of CO2 released from organic acids. During night time, organic acids are synthesized in plenty with the help of CO2 released in respiration and the CO2 entering from the atmosphere through the open stomata. Thus, the CO2 in dark acts as survival value to these plants.
Comparison of the plants of C3 and C4 cycle



Factors affecting photosynthesis
Factors influencing photosynthesis can be divided into external factors, which come from the plant's environment, and internal factors, which relate to the plant's characteristics and processes.
Here, we'll focus on the external factors:
- Light:
- Light Intensity: Photosynthesis rates are directly proportional to light intensity. However, extremely high light levels can reach a saturation point, where they no longer enhance photosynthesis.
- Light Quality (Wavelength): Photosynthesis occurs in the visible light spectrum (between 400 and 700 nm), with red light being most effective, followed by blue light. Green, infrared, and ultraviolet light do not support photosynthesis.
- Light Duration: Longer periods of light exposure, as seen in tropical regions, favor photosynthesis.
- Carbon Dioxide (CO2): Increased CO2 concentration at optimal temperature and light levels enhances photosynthesis. However, very high CO2 levels can become toxic and inhibit the process.
- Temperature: Photosynthesis rates increase with rising temperatures up to a certain point (around 40 ºC). Beyond this temperature, photosynthesis declines due to enzyme denaturation. The temperature requirements vary among plant species.
- Water: While water is a raw material for photosynthesis, its effect on the process is mostly indirect. Water scarcity can lead to reduced photosynthesis as cells become flaccid.
- Oxygen: An increase in oxygen concentration, known as the Warburg effect, can inhibit photosynthesis in many plants, particularly C3 plants. This is linked to photorespiration, where oxygen interferes with key metabolic reactions.
- Mineral Elements: Essential minerals such as Mg, Fe, Cu, Cl, Mn, and P play critical roles in photosynthesis reactions. Deficiencies in these nutrients can reduce photosynthesis.
- Chlorophyll Content: Chlorophyll is essential for capturing light energy. Plants with adequate chlorophyll content exhibit efficient photosynthesis, while chlorotic (yellowing) leaves with reduced chlorophyll have inefficient photosynthesis.
- Leaf Characteristics: Leaf size, chlorophyll content, stomatal number, leaf orientation, and leaf age all influence photosynthesis. Maximal photosynthetic activity is typically observed in fully functional, mature leaves.
- Carbohydrates: Accumulated carbohydrates that are not translocated can reduce photosynthesis and increase respiration. Carbohydrates are often converted to starch, which accumulates in chloroplasts and can hinder photosynthesis.
- Phytohormones: Certain plant hormones, such as gibberellic acid and cytokinins, can influence photosynthetic rates by affecting carboxylating activity. For instance, kinetin has been shown to increase photosynthesis within a short time after treatment.
Photorespiration
Photorespiration is a metabolic process that occurs in the presence of light and involves excessive respiration in green plant cells. It was discovered by Decker in 1955 and is also known as the C2 cycle because it involves a two-carbon compound called glycolic acid as a substrate. While respiration normally occurs both in light and dark conditions, photorespiration specifically takes place when light is present, and it is often referred to as "dark respiration."
Several factors influence photorespiration, including temperature and oxygen concentration. Photorespiration is most active at temperatures between 25 and 30 ºC and is closely tied to oxygen levels. This process involves three cell organelles: chloroplasts, peroxisomes, and mitochondria. Notably, it is observed in plants such as cotton, pulses, capsicum, peas, tomatoes, petunias, soybeans, wheat, oats, paddy rice, and chlorella but is absent in grasses.
- The mechanism of photorespiration is as follows:
- In the presence of excess oxygen and low carbon dioxide (CO2), ribulose 1,5-diphosphate, produced during photosynthesis in chloroplasts, is split into two compounds: 2-phosphoglycolic acid and 3-phosphoglyceric acid by the enzyme ribulose 1,5-diphosphate oxygenase.
- The 3-phosphoglyceric acid enters the Calvin cycle, a crucial process in photosynthesis.
- In the next step, a phosphate group is removed from 2-phosphoglycolic acid by the enzyme phosphatase, forming glycolic acid.
- Glycolic acid exits the chloroplast and enters the peroxisome, where it combines with oxygen to produce glyoxylic acid and hydrogen peroxide, catalyzed by the enzyme glycolic acid oxidase. Hydrogen peroxide is toxic but is broken down into water and oxygen by the enzyme catalase. This process involves the oxidation of glycolic acid and is sometimes referred to as glycolate metabolism.
- Glyoxylic acid is converted into glycine by adding an amino group with the enzyme amino transferase.
- Glycine is transported from the peroxisome to the mitochondria, where two molecules of glycine combine to form serine, releasing carbon dioxide and ammonia.
- An amino group is removed from serine to produce hydroxypyruvic acid with the enzyme transaminase.
- Hydroxypyruvic acid is reduced with the help of NADH to form glyceric acid in the presence of the enzyme alpha hydroxyl acid reductase.
- Finally, glyceric acid is phosphorylated with ATP by the enzyme kinase to regenerate 3-phosphoglyceric acid, an intermediate in the Calvin cycle used for carbohydrate production during photosynthesis.
It's worth noting that the entry of 3-phosphoglyceric acid into the chloroplast for carbohydrate production has been suppressed in some plants due to the increased carbon dioxide content in the atmosphere. This suppression is a response to reduced photorespiration caused by higher atmospheric CO2 levels.
Significance of photorespiration
The significance of photorespiration in plants can be summarized as follows:
- Classification of Plants: Photorespiration serves as a valuable characteristic for classifying plants. It is typically found in C3 plants, where the initial carbon fixation occurs via the Calvin cycle and photorespiration plays a significant role. In contrast, C4 plants have developed mechanisms to minimize photorespiration, making it largely absent in these plants. This distinction between C3 and C4 plants is important in plant taxonomy and understanding their adaptations to different environmental conditions.
- Maintenance of CO2 Levels: Photorespiration results in the evolution of carbon dioxide (CO2). This release of CO2 prevents the complete depletion of CO2 in the vicinity of chloroplasts, which can occur during the Calvin cycle of photosynthesis. This is crucial because CO2 is a necessary substrate for photosynthesis, and maintaining adequate levels of it ensures the continued functioning of the photosynthetic process.
- Removal of Unwanted Byproducts: Photorespiration involves the oxidation of glycolic acid, which is an unwanted byproduct of photosynthesis. By converting glycolic acid into carbohydrate and releasing the remainder as CO2, photorespiration helps the plant dispose of this metabolic waste product.
- Energy Consumption: Photorespiration consumes energy in the form of ATP and reduced nucleotides (NADH and NADPH). This is in contrast to normal respiration, which yields ATP and reduced nucleotides. While photorespiration is energetically costly for the plant, it plays a role in maintaining metabolic balance and preventing damage under specific conditions.
- Historical Significance: It is believed that photorespiration was more prevalent in earlier times when atmospheric CO2 levels were lower. As atmospheric CO2 concentrations have increased due to human activities, some plants have developed adaptations to reduce photorespiration and optimize their photosynthetic efficiency. This evolution reflects the changing environmental conditions on Earth.
In summary, photorespiration is a significant process in plant biology with implications for plant classification, CO2 regulation, waste product removal, energy utilization, and adaptation to changing environmental conditions. While it may be less favorable for plant growth compared to regular respiration, it plays a role in maintaining metabolic equilibrium and has historical relevance in plant evolution.
|
179 videos|140 docs
|
FAQs on Photosynthesis: Photochemical reactions, Photophosphorylation & Carbon fixation pathways - Botany Optional for UPSC
| 1. What is a photochemical reaction? |  |
| 2. How do photochemical reactions contribute to photosynthesis? |  |
| 3. What is photophosphorylation and its significance in photosynthesis? |  |
| 4. What is the Calvin cycle and its role in photosynthesis? |  |
| 5. Can you explain the dark reaction or Blackman's reaction in photosynthesis? |  |

|
Explore Courses for UPSC exam
|

|

























