Development of Genetics | Botany Optional for UPSC PDF Download
| Table of contents |

|
| Overview of Drosophila Development |

|
| Coordinate Genes |

|
| Zygotic Genes |

|
| Homeotic Genes |

|
Overview of Drosophila Development
We will primarily focus on Drosophila in our discussion of developmental genetics because the research conducted on this model organism has provided comprehensive insights. Many fundamental principles discovered in Drosophila research have broader applications to development in various animals, including humans.
Drosophila development can be summarized as a process of precisely defining the position of each part of the embryo along both the anterior-posterior and dorsal-ventral axes. This positional information serves as a guide for cells to determine their future identities. This process involves a series of genes with distinct roles, some of which are specific to embryonic development and are not active once embryogenesis is completed.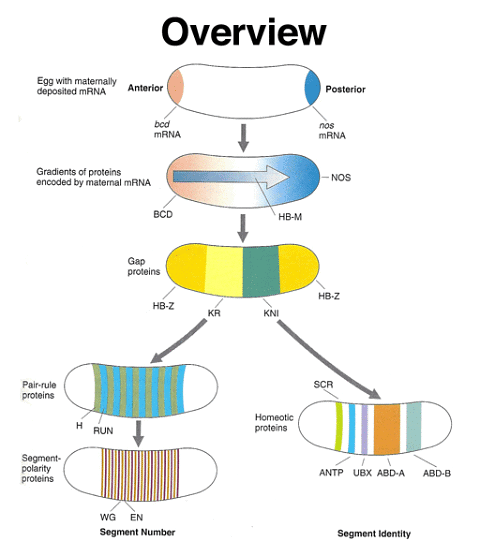
To delve into more detail, there are maternal coordinate genes that establish positional information within the egg. These genes create gradients of morphogens within the egg, and their information is interpreted by the gap genes, which are the first zygotic developmental genes to be activated. Gap genes are expressed in broad regions encompassing multiple adjacent future segments. Following this, the pair-rule genes are expressed in a pattern that alternates between future segments. These genes regulate the expression of segment-polarity genes, which define specific regions within individual segments. Concurrently, homeotic genes are expressed in a manner that assigns a unique identity to each segment.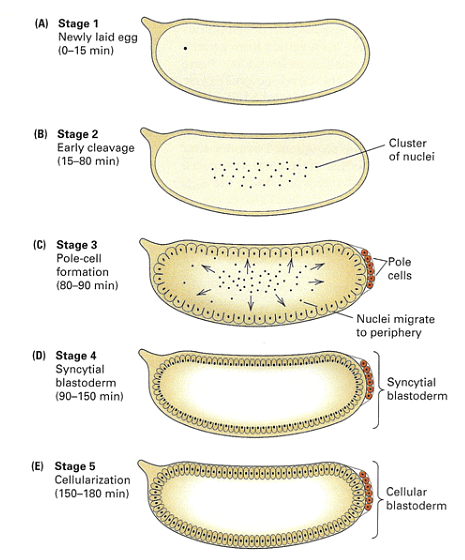
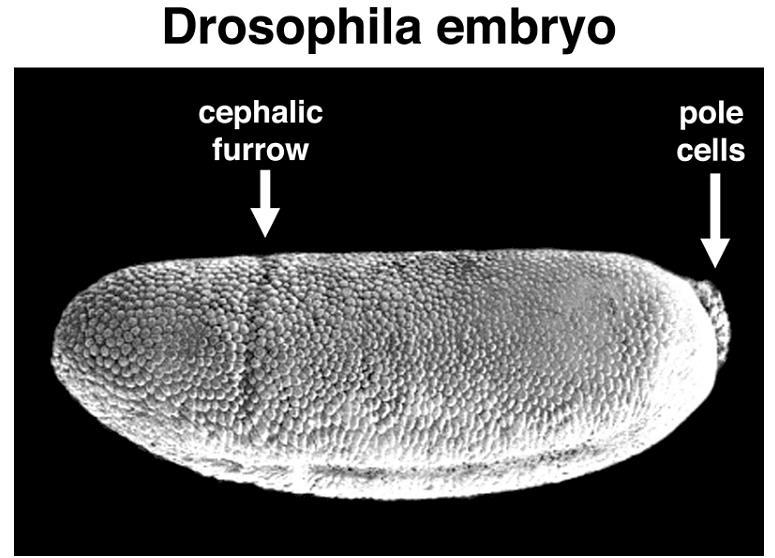
Early Drosophila development is distinctive in that initial nuclear divisions occur rapidly, approximately every ten minutes, without cell division. Subsequently, cells migrate to the embryo's periphery. The first nuclei reaching the posterior pole become pole cells, which will give rise to the future germ line. Shortly afterward, all other peripheral nuclei undergo cellularization simultaneously.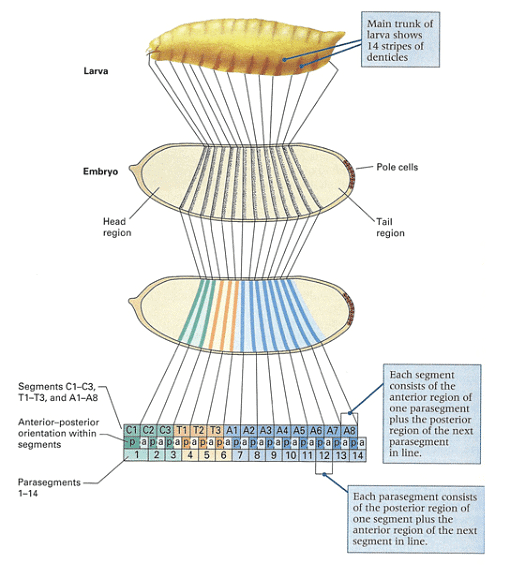
As depicted in the figure, the pattern of the first-instar larva, resulting from embryonic development, can be mapped to specific regions within the cellular blastoderm. The larva exhibits fourteen stripes of denticle belts, reflecting the three head, three thoracic, and eight abdominal segments observed during embryogenesis.
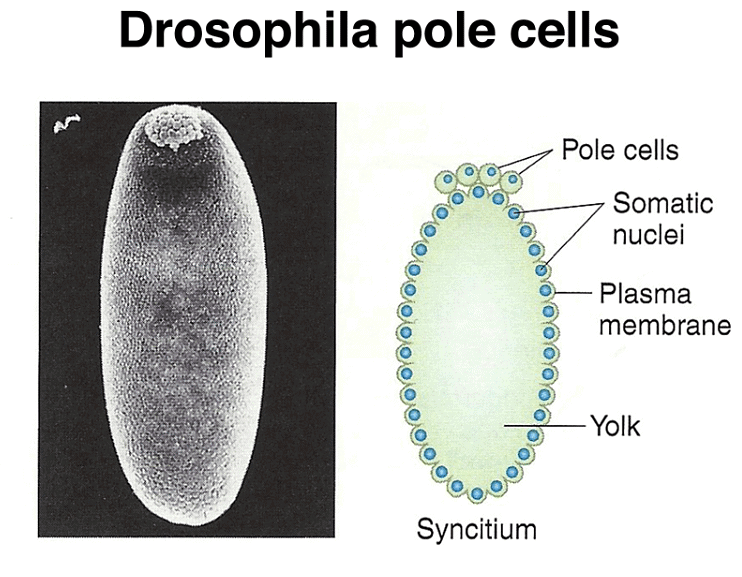
Pole cell formation
Pole cells, the future germ line, are set aside early in development. The image below shows a scanning electron micrograph of an early embryo with the posterior pole at the top of the image. The pole cells incorporate polar granules, particles of RNA and protein that are deposited in the egg by the mother during oogenesis.
Coordinate Genes
The developmental process in Drosophila involves the establishment of precise positional information within the embryo's anterior-posterior and dorsal-ventral axes. This information is crucial for cells to determine their identities, and it is achieved through the action of specific proteins. Two key proteins involved in this process are bicoid (BCD) and nanos (NOS), both of which act as transcription factors.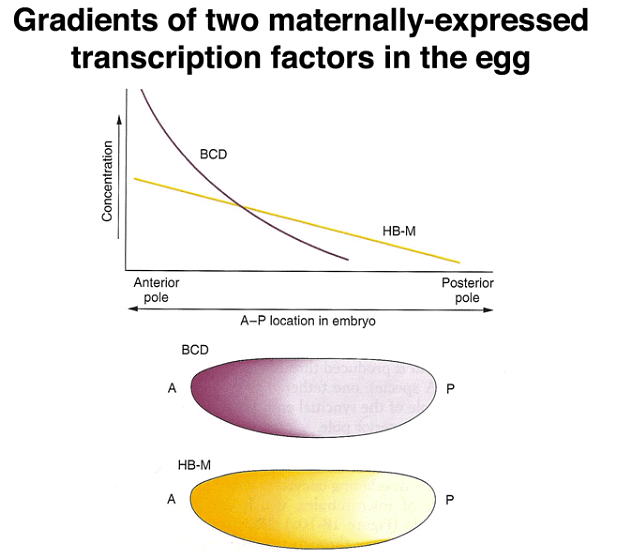
Experimental data reveals that localized RNA molecules at the anterior pole of the egg lead to the synthesis of BCD and NOS proteins through translation. The resulting proteins create gradients along the anterior-posterior axis, and the distinct patterns of these gradients allow cells to determine their positions. Importantly, the mRNA is anchored in place, while the protein is free to diffuse within the embryo.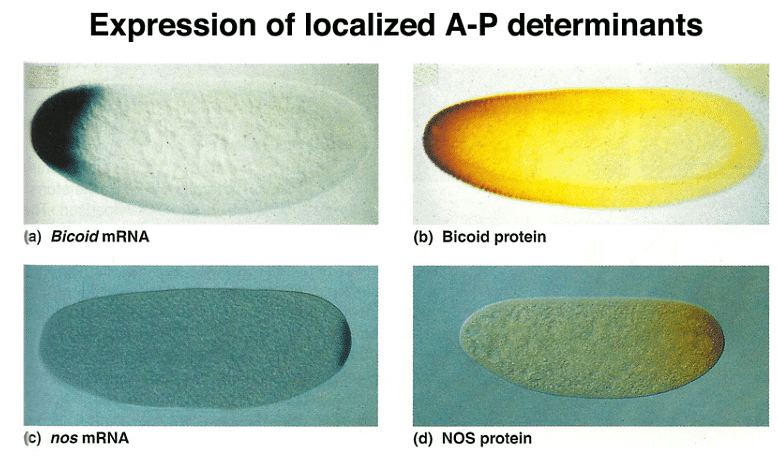
Further experiments demonstrate that the information needed to localize and tether bcd mRNA to the anterior pole resides in its 3' untranslated region (UTR). Combining the 5' UTR and coding region of nanos with the 3' UTR of bcd leads to the localization of the hybrid mRNA to the anterior pole and affects development.
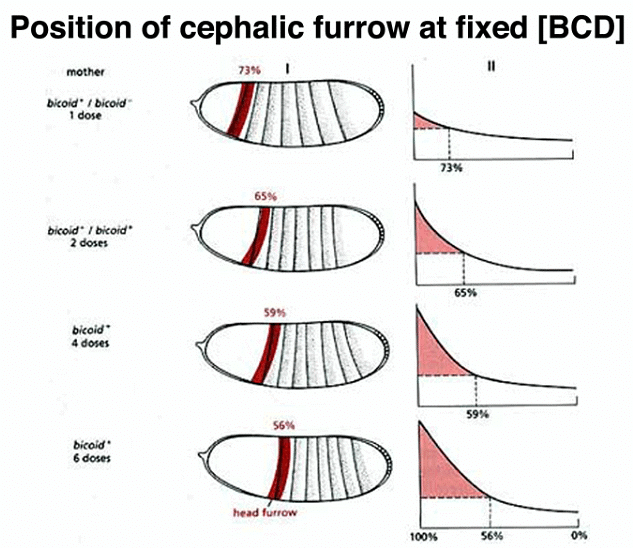
Manipulating the dosage of the normal bcd gene in the mother influences the amount of BCD protein synthesized, resulting in changes in the cephalic furrow's position, a boundary between future cephalic and thoracic structures.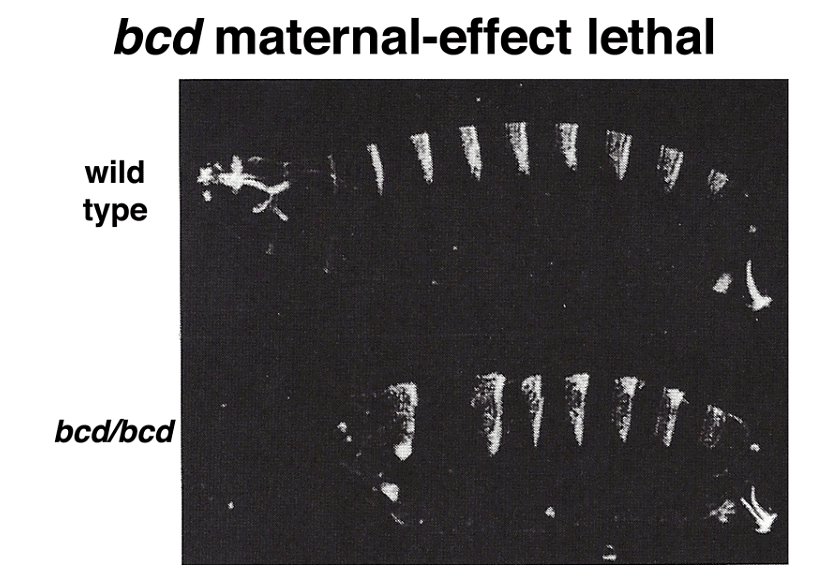
Moreover, experiments involving the transfer of cytoplasm from a normal egg or bcd mRNA transcribed from a cloned copy of the bcd gene into embryos from bcd/bcd mothers demonstrate the ability to restore anterior structures in these embryos.
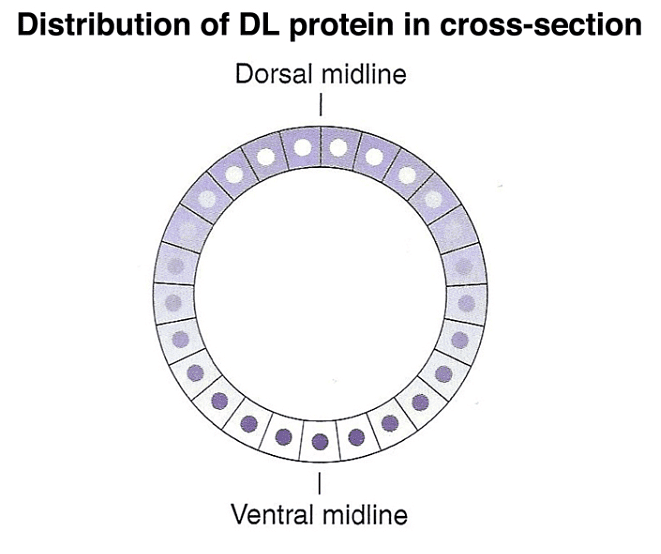
In addition to the anterior-posterior axis, a gradient of another morphogen, the dorsal protein (DL), specifies the dorsal-ventral axis. The DL protein's concentration gradient along this axis helps define cell positions.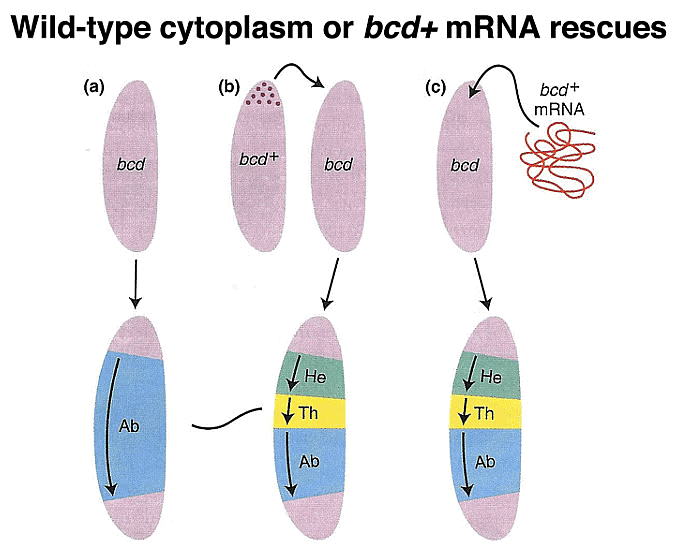
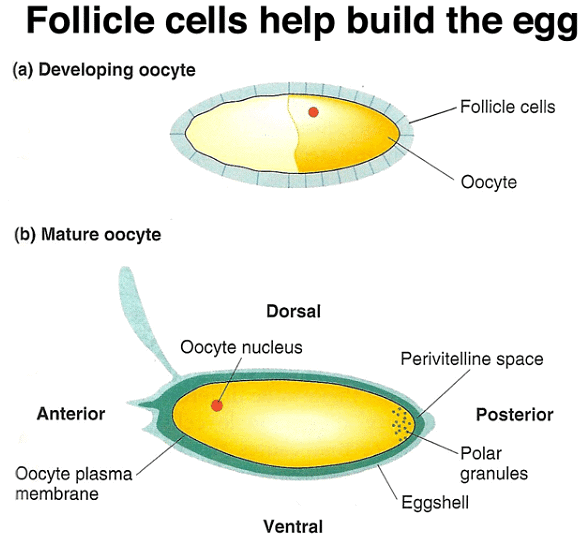
Follicle cells play a role in forming the perivitelline space and eggshell during egg development.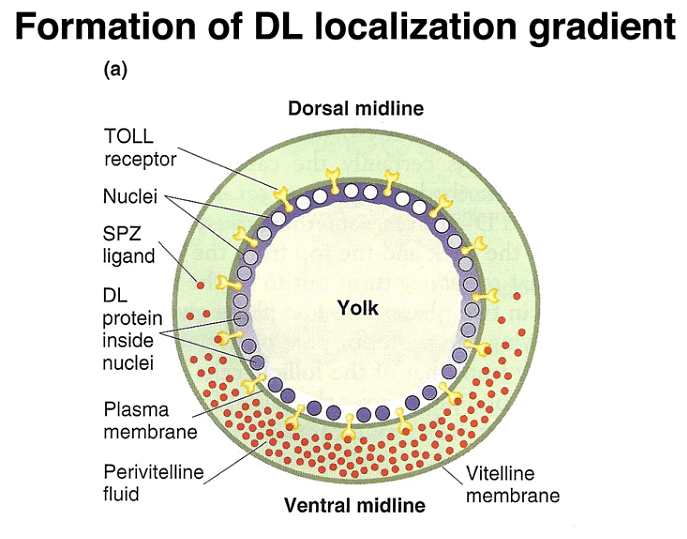
During the cellular blastoderm stage, a concentration gradient of another morphogen, the SPZ protein, is observed, with the highest concentration at the ventral midline. The SPZ protein binds to the TOLL receptor, leading to the nuclear localization of DL.
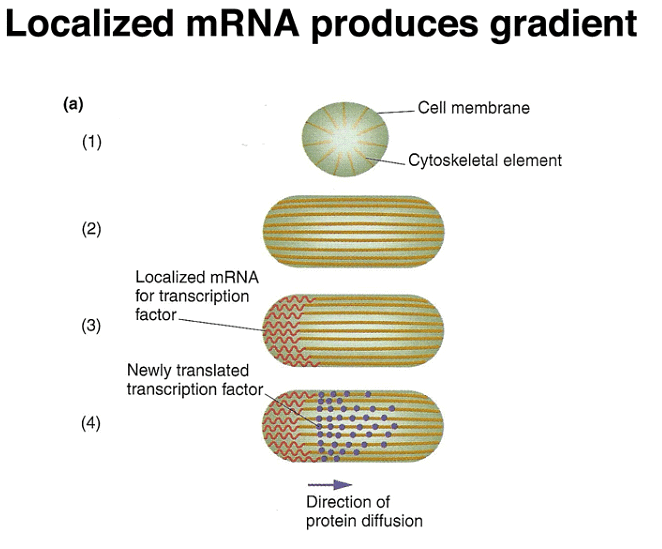
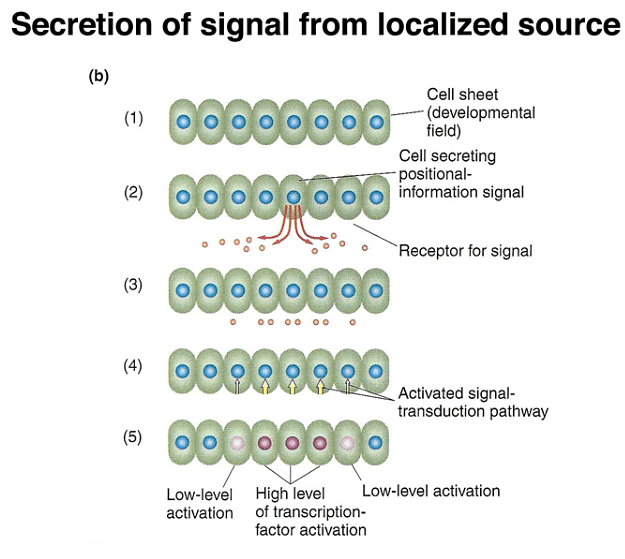
These findings reveal a common mechanism where tethered mRNA translation leads to the establishment of protein concentration gradients, as demonstrated by the example of bcd. Additionally, later in development, paracrine signaling involves cells secreting positional-information proteins, which neighboring cells interpret based on the signal's concentration.
Zygotic Genes
During Drosophila embryo development, as cellularization occurs, each cell receives the proteins present in its specific cytoplasmic position. This process transforms the concentration gradients of morphogen proteins within the early egg into distinct protein concentration levels within individual cells.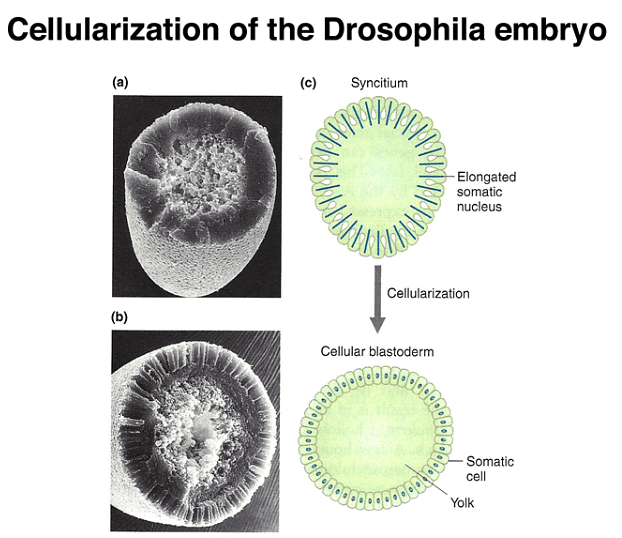
As development progresses, a segmentation pattern emerges, which corresponds to the segmentation seen in the first-instar larva. This pattern includes the thoracic (T1 - T3) and abdominal (A1 - A8) segments, as well as head segments, although the latter become less visible in the larva due to a process called head involution.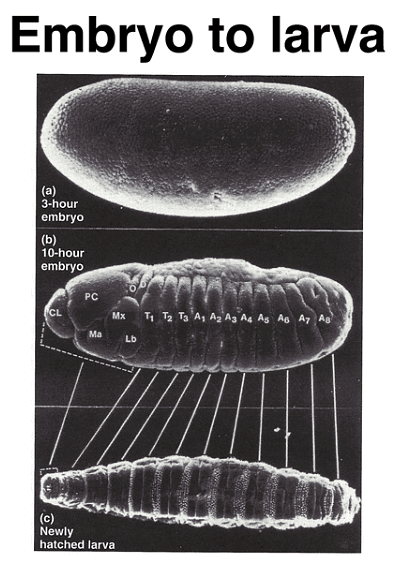
Following the initial stages of Drosophila development and the formation of the cellular blastoderm, the zygotic genome becomes active, and transcription of zygotic genes begins. Through a genetic screen that identified recessive mutations hindering embryonic development, various types of zygotically-active genes were identified. These genes allow embryos to progress to the stage where they develop a larval cuticle, making it possible to analyze defects in embryonic patterning.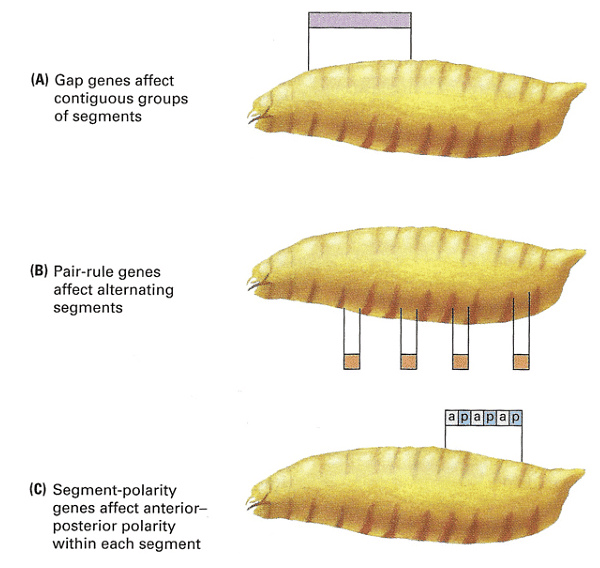
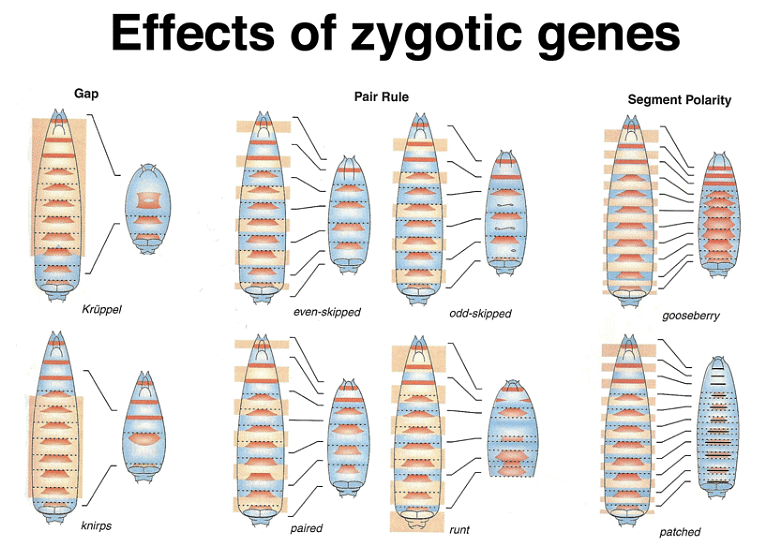
Zygotically-acting genes can be broadly categorized into three groups: Gap genes, pair-rule genes, and segment-polarity genes. Gap genes are expressed early in broad bands along the anterior-posterior axis and are crucial for specifying segments. Pair-rule genes, on the other hand, cause defects in alternating segments when mutated and are expressed in a striped pattern along the axis.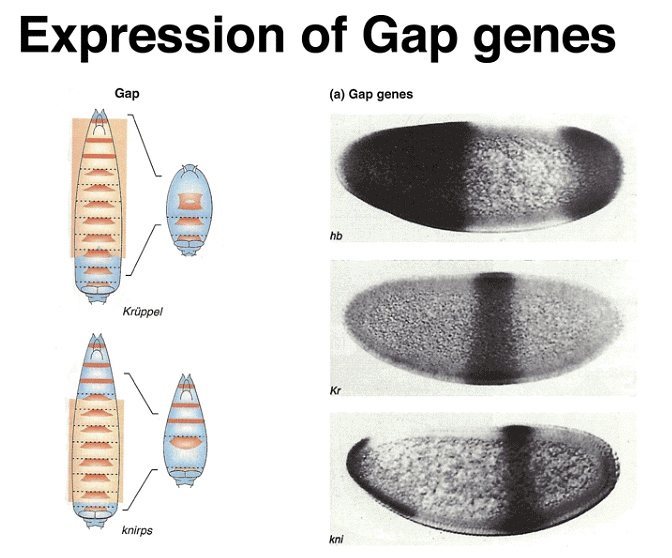
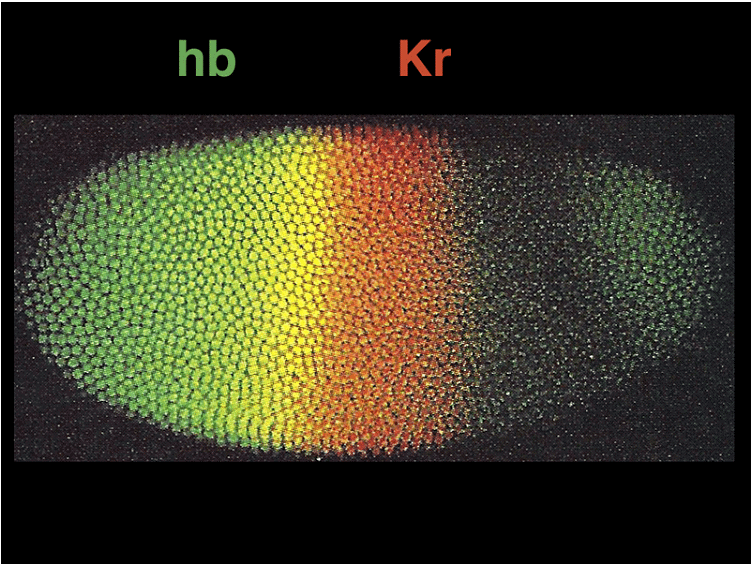
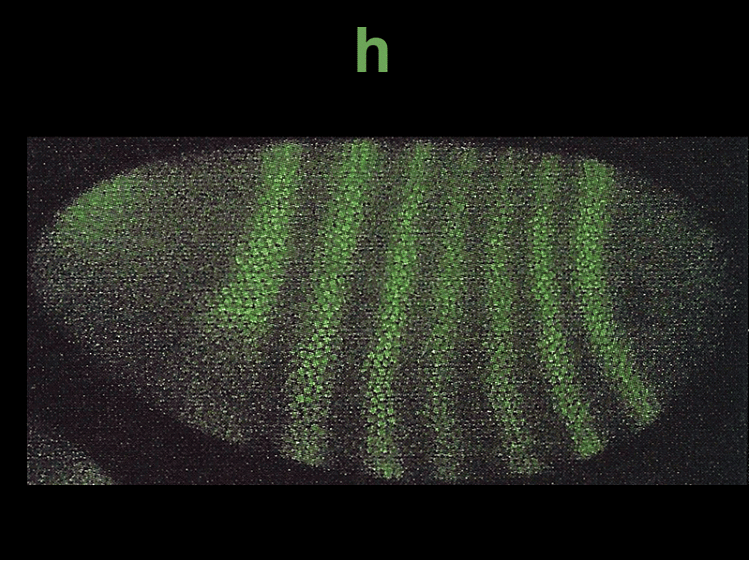
The expression patterns of these genes can be visualized using techniques like in situ hybridization and immunofluorescence. Gap gene expression, for example, can be seen through the presence of proteins like hb, Kr, and kni, while pair-rule gene expression can be observed using markers like eve, ftz, and h.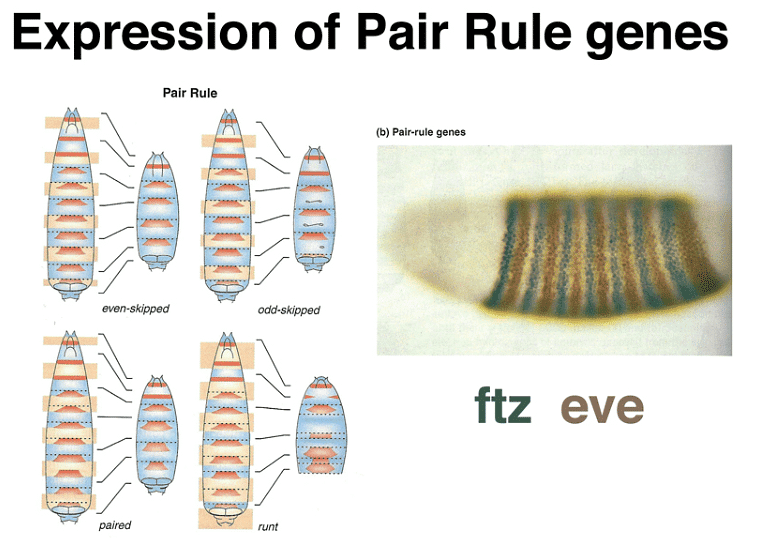
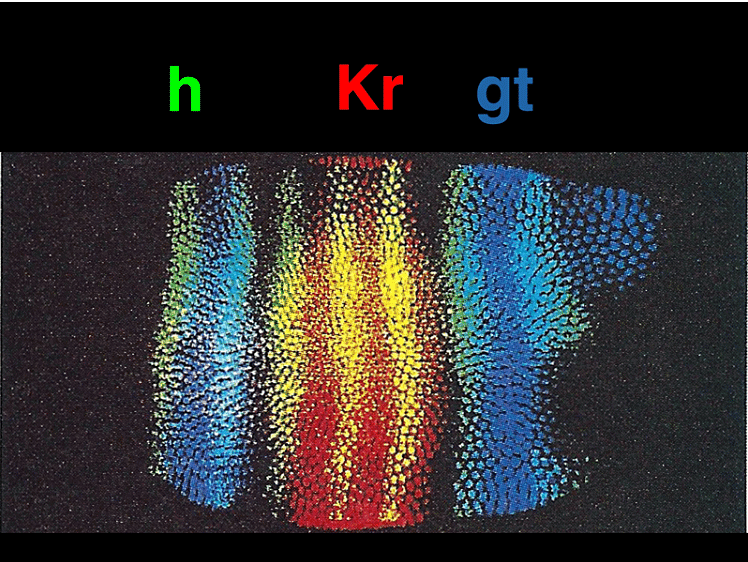

Finally, the segment polarity genes are activated later in development and help define the polarity and boundaries within individual segments. These genes play a role in stabilizing their own expression through autoregulation and contribute to the precise patterning of the embryo. Additionally, paracrine signaling among cells sharpens segment boundaries by allowing neighboring cells to communicate their identities to one another.
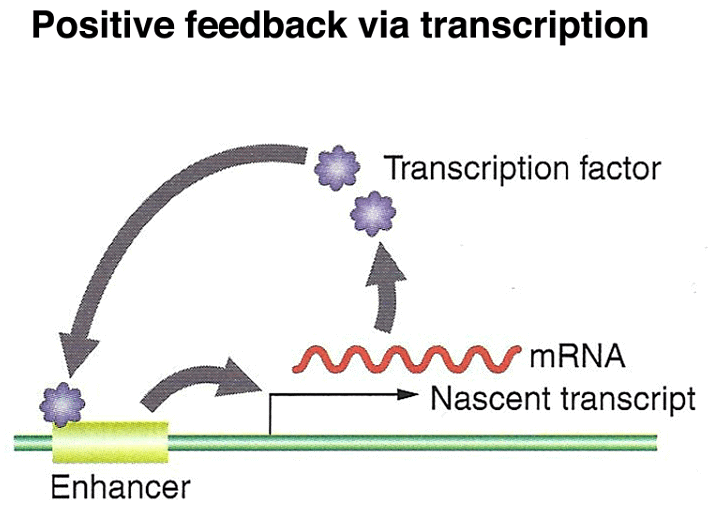
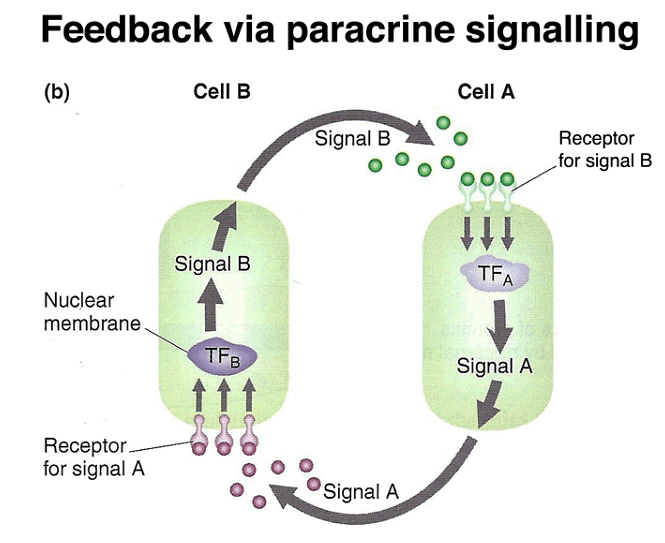
Homeotic Genes
In the course of Drosophila development, alongside the gap, pair-rule, and segment polarity genes, another crucial set of genes called homeotic genes comes into play. Homeotic genes are responsible for determining the identity of each segment in the developing embryo. When these genes are mutated, they can lead to homeotic transformations, which are dramatic changes in the identity of body parts.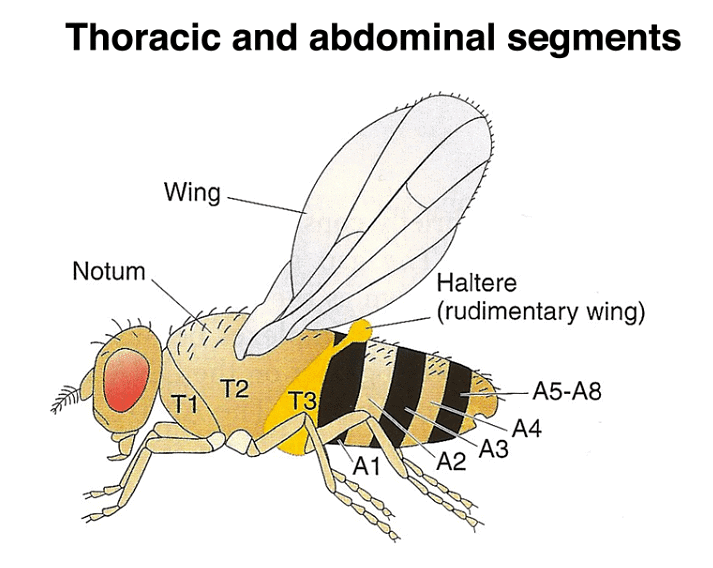
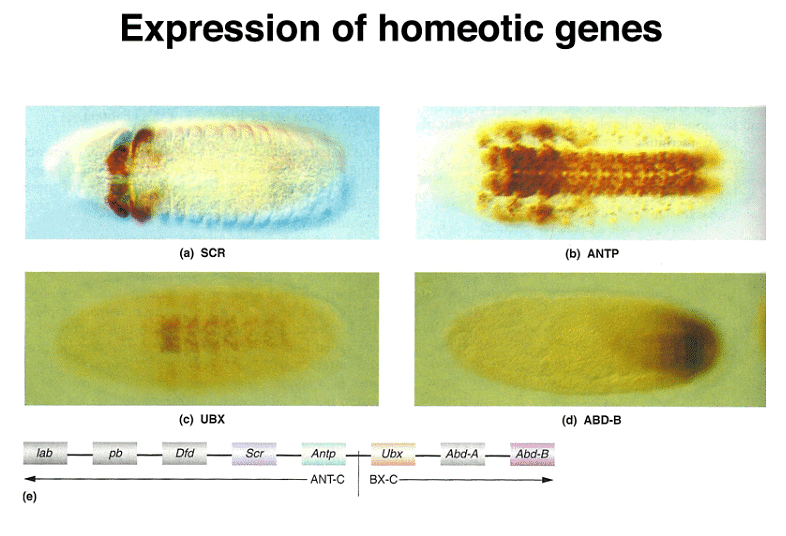
To understand the significance of these mutations, it's important to know the adult anatomy of Drosophila. There are three thoracic and eight abdominal segments. Unlike certain primitive arthropods that have both gill and leg appendages on each segment, Drosophila has lost all appendages on its abdominal segments. In contrast, the thoracic segments each have legs, with the second thoracic segment bearing wings and the third thoracic segment having halteres, which are vestigial wings. Notably, Diptera (flies), including Drosophila, have only one pair of wings on the second thoracic segment, unlike dragonflies and other winged insects that have two pairs of wings.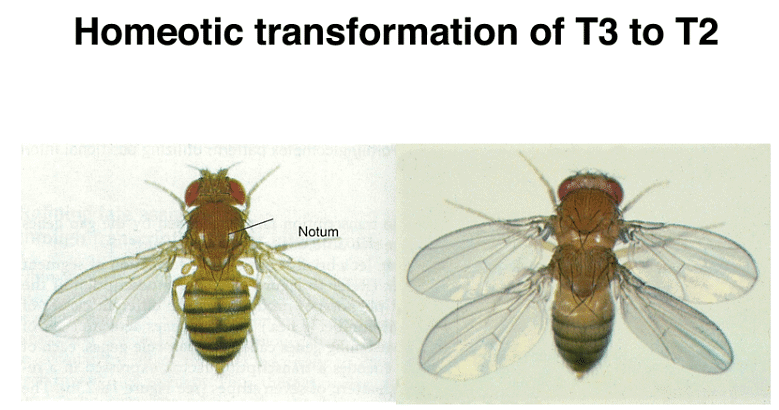
Homeotic genes are expressed across multiple segments, and the concentration of their protein products specifies the identity of each segment. Mutations in these genes can lead to remarkable phenotypic changes. For instance, mutations in the bithorax genes can transform the third thoracic segment into a second thoracic segment, resulting in an extra set of wings.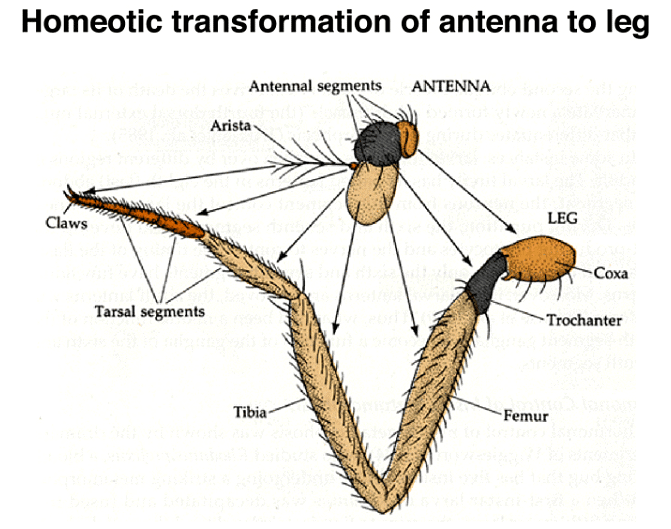
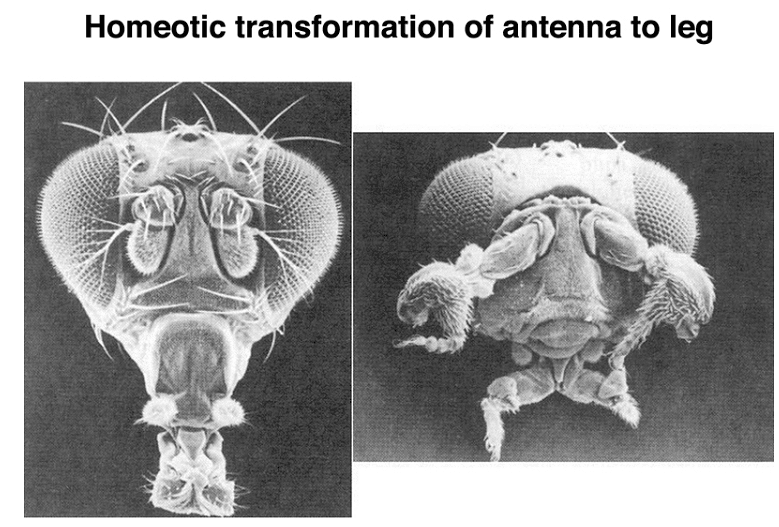
Another example of a homeotic transformation is seen in mutations of the Antennapedia gene, which can transform antennae into legs.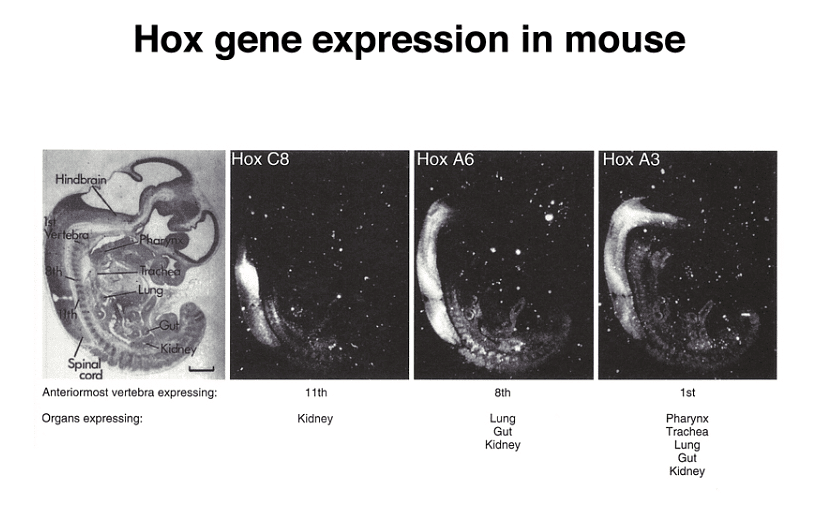
Remarkably, even in cases of partial transformations, where an appendage retains some of its original characteristics while becoming a different appendage, the proximal-to-distal positional information is maintained. This means that each part of the transformed appendage "knows" its location along the proximal-to-distal axis, even if it has been converted to a different appendage type.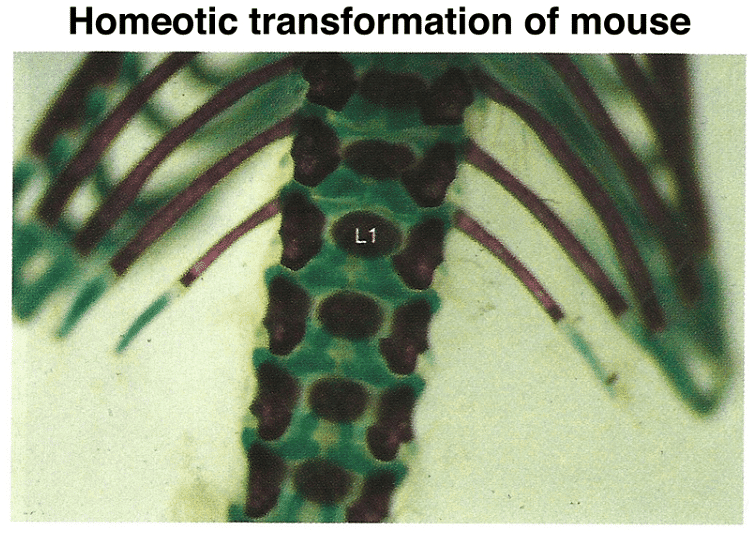
When asked how a single gene could bring about such profound transformations, the answer lies in the fact that homeotic genes encode transcription factors that control the expression of numerous other genes. Their regulatory role extends across many downstream genes.
Importantly, homeotic genes are not unique to Drosophila; they are also found in vertebrates, including mice and humans. These genes are known as Hox (homeobox) genes in vertebrates. While Drosophila has a single cluster of Hox genes, mammals have four clusters, a reflection of genome duplication early in vertebrate evolution.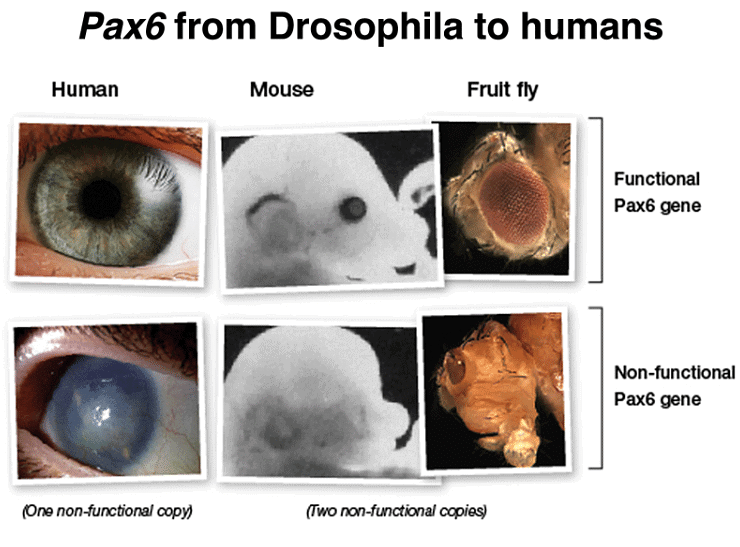
Mutations in specific Hox genes can lead to homeotic transformations in mice, such as the development of extra ribs on certain vertebrae.
Furthermore, there is another type of developmental gene that shares similarities with homeotic genes. These genes, like Pax6, encode transcription factors that control the expression of many target genes. Mutations in Pax6 can result in the absence of eye development in both mice and humans. Intriguingly, when the human Pax6 gene is expressed in Drosophila mutants lacking the eyeless gene (the fly equivalent of Pax6), it can restore eye development. This demonstrates the remarkable conservation of gene function across the 600 million years of evolution that separate humans from Drosophila.
Summary
The figure below summarizes the different types of developmental genes that we have discussed. The coordinate genes are expressed maternally to establish morphogen gradients in the egg. The concentration of various morphogens is interpreted by a set of zygotically-acting gap genes, which have patterns of expression that span several future segments. The positional information is further refined by pair-rule and segment polarity genes that define a pattern of segmentation. Expression of homeotic genes across these segments establishes a set of segmental identities that guides differentiation.

|
179 videos|143 docs
|
FAQs on Development of Genetics - Botany Optional for UPSC
| 1. What are coordinate genes in Drosophila development? |  |
| 2. What are zygotic genes in Drosophila development? |  |
| 3. What are homeotic genes in Drosophila development? |  |
| 4. How do coordinate genes influence Drosophila development? |  |
| 5. What is the significance of Drosophila development in genetics research? |  |















