Important Diagrams: Structure of the Atom | Science Class 9 PDF Download
Q1: Answer the following questions based on the diagram given below: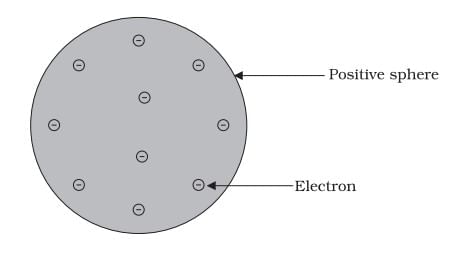
(i) What does Thomson's model of an atom resemble?
Ans: Thomson's model of an atom is likened to a plum pudding, where the positive charge represents the "pudding," and the electrons are the "plums" embedded within it.
(ii) How does Thomson's model describe the distribution of charge within an atom?
Ans: Thomson's model suggests that the positive charge is spread uniformly throughout the atom, with electrons embedded within it, dispersing the negative charge.
(iii) What is the nature of the particles in Thomson's atomic model?
Ans: The atom in Thomson's model consists of positively charged particles (representing the pudding or sphere) and negatively charged particles (representing the plums or electrons).
(iv) What is the role of electrons in Thomson's atomic model?
Ans: Electrons in Thomson's model are depicted as negatively charged particles embedded within the positively charged sphere, providing a balanced charge for the atom.
(v) How does Thomson's model differ from the current understanding of atomic structure?
Ans: Thomson's model suggests that electrons are distributed uniformly within a positively charged sphere, unlike the modern understanding, where electrons orbit the nucleus in specific energy levels.
Q2: Answer the following questions based on the diagram given below: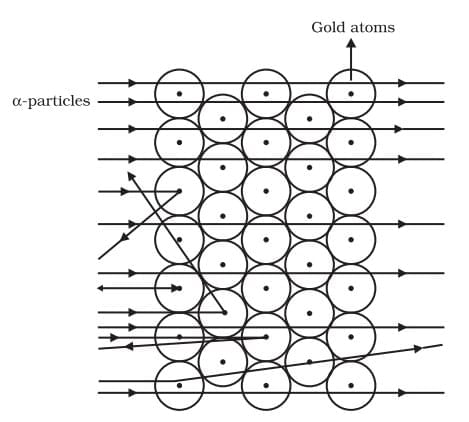
(i) What were the unexpected observations from the α-particle scattering experiment?
Ans: The unexpected observations from the α-particle scattering experiment were:
- Most fast-moving α-particles passed straight through the gold foil.
- Some α-particles were deflected by small angles.
- Approximately one out of every 12,000 particles rebounded.
(ii) What did Rutherford conclude from the α-particle scattering experiment regarding the space inside an atom?
Ans: Rutherford concluded that:
- Most of the space inside the atom is empty, as most α-particles passed through the gold foil without deflection.
- The positive charge of the atom occupies very little space based on the few deflected particles.
- A tiny fraction of α-particles being deflected by 180 degrees suggests that all the positive charge and mass of the gold atom are concentrated in a very small volume within the atom.
(iii) How did Rutherford calculate the radius of the nucleus based on the α-particle scattering experiment?
Ans: Rutherford calculated the radius of the nucleus by comparing the scattering data. He found that the radius of the nucleus is about 10,000 times smaller than the radius of the entire atom.
(iv) What were the features of Rutherford's nuclear model of an atom?
Ans: Rutherford's nuclear model of an atom had the following features:
- The atom has a positively charged centre called the nucleus, containing nearly all the atom's mass.
- Electrons revolve around the nucleus in circular orbits.
- (The size of the nucleus is significantly smaller compared to the overall size of the atom.
(v) What was a major drawback of Rutherford's model of the atom, and why was it considered unstable?
Ans: The major drawback was that the model proposed electrons revolving in circular orbits, which should lead to continuous energy loss due to radiation during acceleration. This instability would cause the electrons to lose energy and eventually spiral into the nucleus, making atoms highly unstable and incompatible with the stable matter we observe.
Q3: Answer the following questions based on the diagram given below: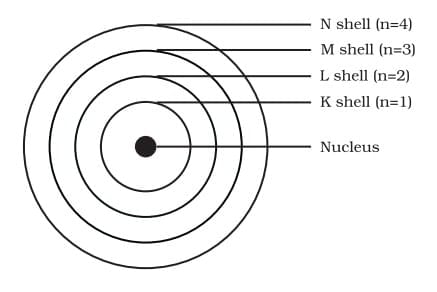
(i) What do the concentric circles around the nucleus represent in the given diagram?
Ans: The concentric circles represent energy shells or orbits in which electrons revolve around the nucleus. Each shell is denoted by a specific letter: K (n=1), L (n=2), M (n=3), and N (n=4).
(ii) Why are electrons placed in different shells rather than all being in one shell?
Ans: Electrons are placed in different shells because each shell has a maximum capacity determined by the 2n² rule. Electrons fill the shells in order of increasing energy, starting with the shell closest to the nucleus. This arrangement provides stability to the atom.
(iii) What is the maximum number of electrons that can be present in the third orbit or M-shell?
Ans: The maximum number of electrons that can be present in the third orbit or M-shell is 18.
Using the formula 2n², for n = 3:
Maximum electrons = 2 × (3)² = 2 × 9 = 18 electrons.
(iv) How many electrons can the outermost orbit of an atom hold?
Ans: The outermost orbit of an atom can hold a maximum of 8 electrons.
(v) In what manner are electrons accommodated in different shells of an atom?
Ans: Electrons are not accommodated in a given shell unless the inner shells are filled. In other words, the shells are filled in a step-wise manner.
Q4: Find out the valency of the atoms represented by the Fig. (a) and (b).
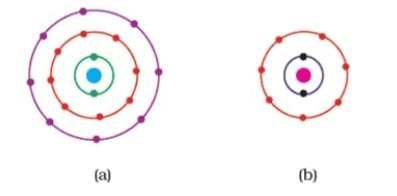
Ans: Atom (a) has zero valency because it has established a stable configuration with 8 electrons in the valence shell. The valency of atom (b) is 1 since the valence shell contains 7 electrons. To obtain a stable (octet) configuration, atom (b) can take one additional electron.
Q5: What information do you get from the Fig. about the atomic number, mass number and valency of atoms X, Y and Z? Give your answer in a tabular form.
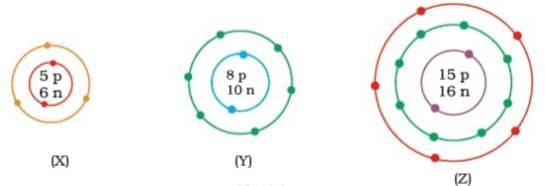
Ans: Atomic number, mass number and valency of atoms X, Y and Z.
| Atomic no | Mass no | Valency | |
|---|---|---|---|
| X | 5 | 11 | 3 |
| Y | 8 | 18 | 2 |
| Z | 15 | 31 | 3.5 |
Q6: The given figure depicts the atomic structure of an atom of an element ‘X’. Write the following information about the element ‘X’.
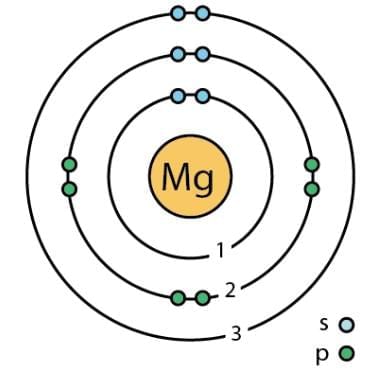 (a) Atomic number of ‘X’
(a) Atomic number of ‘X’
(b) Atomic mass of ‘X’
(c) Valence electrons
(d) Valency of ‘X’
(e) ‘X’ should be metal or non-metal.
Ans: (a) 12
(b) 24
(c) 2
(d) 2
(e) Metal
|
84 videos|478 docs|60 tests
|
FAQs on Important Diagrams: Structure of the Atom - Science Class 9
| 1. What is the structure of an atom? |  |
| 2. What are protons, neutrons, and electrons? |  |
| 3. How are protons, neutrons, and electrons arranged in an atom? |  |
| 4. What determines the identity of an atom? |  |
| 5. What happens if an atom gains or loses electrons? |  |





















