Sorting Materials into Groups Chapter Notes | Eureka Plus Class 6: Book Solutions, Notes & Worksheets PDF Download
Introduction
To simplify the concept of sorting things into groups, imagine organizing objects by their shape and behavior, like separating items into solids, liquids, and gases. This categorization helps us recognize characteristics and potential interactions effectively.
Benefits of Sorting Things into Groups
- Understanding States of Matter: Grouping items as solids, liquids, or gases based on their shape and behavior. Solids like an iron pin, sea salt, and chart paper maintain a fixed shape. Liquids can flow and take the shape of their container, while gases spread to fill available space.
- Preventing Reactions: Recognizing that certain items shouldn't be stored together due to potential reactions. For instance, salt and iron can't be kept together as salt can cause the iron to rust.
- Importance of Classification: Classifying objects based on their features, properties, or characteristics. Using plants as an example, they are grouped as herbs, shrubs, and trees according to their stem nature, size, and lifespan.
- Facilitating Understanding: Understanding the nature of items by grouping them helps in various fields. For plants, knowing their classification aids in cultivation and care practices.
Materials, Properties and Uses

Materials are substances that we use for different purposes in our daily lives. They can be natural, like water and wood, or man-made, like plastics and metals. Each material has unique characteristics that determine how we can use it.
- Metals and Plastics:
- Metals: Metals are hard, durable, shiny, have high melting points, and conduct heat and electricity well. Because of these properties, we use metals for various things.
- Plastics: Plastics have their own set of specific uses due to their unique properties.
- Examples of Uses:
- Metals: Utensils, ornaments, machine parts, furniture, construction materials, and more.
- Plastics: Kitchenware, bottles, furniture, electronics components, and more.
- Why Certain Materials for Plates: Materials like glass, steel, plastic, and ceramic are chosen for plates because they don't easily react with food.
- Role of Fine Ceramics: Fine ceramics are a great example of how material properties impact their uses. They are used in computer chips, insulating devices, kitchenware, and even spaceship components because of their hardness, high melting points, and poor conductivity of heat and electricity.
- Understanding Material Properties: Knowing the properties of materials is crucial for using them effectively in various applications.
Properties of Materials
Materials have different properties that make them unique and useful in various ways. Understanding these properties helps us comprehend why materials behave the way they do in different situations.
Appearance
- Distinct Looks: Materials like copper, aluminum, and steel look different from each other. For instance, copper utensils have a different appearance compared to aluminum or steel utensils.
- Lustre: Some materials, especially metals, have a shiny appearance when polished. Rocks like marble and granite also exhibit a distinctive shine when polished.
- Precious Stones: Precious stones like rubies and diamonds are hard, have a pleasant shine, and reflect light when polished. These stones are valuable and are often used in making jewelry.
Hardness
- Hard Materials: Metals, diamonds, plastics, glass, wood, and rocks are examples of hard materials.
- Breakage: Some hard materials like glass and certain types of plastics break easily, while metals, wood, and some plastics are more durable.
- Soft Materials: Materials like paper, wool, cotton, and rubber are soft. Rubber, for example, can be compressed easily.
Transparency
- Transparent Materials: Transparent materials allow light to pass through them completely, enabling us to see through them.
- Examples: Clear glass, air, clean water, and certain types of plastics are transparent materials.
Float or Sink
- Float: Objects like wood, plastic, and pumice stone float on water because they are less dense than water.
- Sink: Objects such as rocks, metal nails, iron balls, and pins sink in water because they are denser than water.
- Density: Density is the mass per unit volume of an object. Objects with a density less than that of a liquid will float on it, while those with greater density will sink.
Heat Conduction
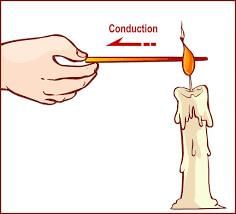
- Definition: Heat conduction is the process of transferring heat from one material to another.
- Good Conductors: Some materials efficiently transfer heat and are known as good conductors. Examples include metals like copper, aluminum, and silver.
- Poor Conductors: Materials that do not transfer heat efficiently are called poor conductors. Examples are wood, plastics, ceramics, and fiberglass.
- Heat Insulators: Poor conductors, also known as insulators, can shield objects from heat. These materials are used for insulation purposes.
- Examples:
- Good Conductor Example: Imagine a metal spoon getting hot when placed in a cup of hot tea. The metal efficiently conducts heat, making the spoon hot to touch.
- Poor Conductor Example: Think of a wooden handle of a cooking utensil. It does not get as hot as the metal parts because wood is a poor conductor of heat.
- Insulator Example: When you hold a cup of hot coffee with a paper sleeve, the sleeve acts as an insulator, preventing your hand from getting burnt by the heat of the coffee cup.
Electrical Conduction
- Definition: Electrical conduction is the transfer of electricity through materials.
- Good Conductors: Materials that efficiently conduct electricity are known as good conductors. Most metals, especially copper and silver, are excellent conductors of electricity.
- Non-Conductors: Materials like ceramics, fiberglass, mica, and plastics do not conduct electricity.
- Insulating Materials: Non-conductors are used to insulate objects from electric currents. They prevent the flow of electricity.
- Examples:
- Good Conductor Example: Copper wires are commonly used to conduct electricity in homes because copper is a good conductor.
- Insulator Example: Consider the plastic casing of an electrical wire. It prevents the electricity from leaking out and shields us from electric shocks.
Soluble and Insoluble Materials
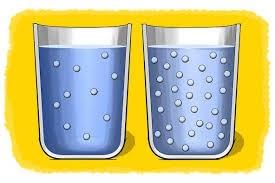
When certain materials can dissolve in water and others cannot, we categorize them as either soluble or insoluble substances. Soluble substances easily dissolve in water, forming what we call water-soluble substances. For example, everyday items like salt, sugar, alum, and honey easily dissolve in water. On the other hand, substances like sand and oils do not dissolve in water and are classified as insoluble.
Soluble Materials
- A substance that can dissolve in water is known as water-soluble. Examples of these include salt, sugar, alum, and honey.
- When a liquid can dissolve in water, we describe it as miscible. Milk is a common example of a miscible liquid.
- When a material dissolves in a liquid, it forms a solution. For instance, when salt dissolves in water, it creates a salt solution, also known as an aqueous solution of salt.
- Aqueous solution refers to a solution in water. Numerous substances, such as salts, fruit juices, oxygen, and carbon dioxide, dissolve in water.
- Oxygen dissolved in water is essential for the survival of plants and animals living in water.
Insoluble Materials
- Materials that do not dissolve in water, like sand and oils, are known as insoluble substances.
- Liquids that do not dissolve in water are termed immiscible in water.
States of Matter
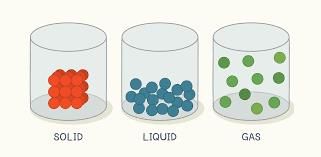
Matter is made up of tiny particles called molecules. The way these molecules are arranged affects the state of matter they form, whether it's solid, liquid, or gas.
Solid State
- In solids like ceramics, silver, and cotton, molecules are tightly packed together.
- Because of this tight packing, molecules in solids cannot move freely, making solids rigid with a definite shape and volume.
- Metals have molecules arranged in an orderly manner, making them good conductors of electricity and heat.
- Wood, on the other hand, has molecules arranged less orderly, which affects its ability to conduct heat.
- Metals can be compressed to some extent under extreme pressure.
Liquid State
- Liquids like nectar and oils have molecules that are closely packed but not as tightly as in solids.
- Molecules in liquids can move around, allowing liquids to flow with no definite shape but a definite volume.
- Even though liquids flow, their molecules stay together, ensuring a constant volume (like a liter of milk always measuring a liter).
- Liquids can be compressed slightly.
Gas State
- Gases are the lightest state of matter, with molecules that are far apart and move freely.
- This free movement allows gases to spread to fill the space they are in, with no definite shape or volume.
- When an incense stick burns, the fragrance spreads because the smoke is a gas that diffuses into the room.
- All gases can be compressed significantly.
Classifying things according to their properties
When we group things together based on what they have in common, it's called classifying. This helps us understand and organize different items more easily.
Key Points
- Classification: Classification means putting things with similar qualities into the same group. For example, metals like iron, aluminum, copper, and gold have common traits, so we classify them together.
- Properties of metals: Most metals are hard, conduct electricity and heat well, have high melting points, and are not easily broken.
- Importance of classification:
- Classification helps us easily understand which substances dissolve in water and form aqueous solutions.
- It assists in choosing the right material for specific needs. For instance, if we need a hard material that conducts electricity well, we know to pick a metal like copper.
|
22 videos|80 docs|16 tests
|
FAQs on Sorting Materials into Groups Chapter Notes - Eureka Plus Class 6: Book Solutions, Notes & Worksheets
| 1. What are the benefits of sorting materials? |  |
| 2. How do properties of materials play a role in sorting them into groups? |  |
| 3. How can sorting materials into groups help in understanding their uses better? |  |
| 4. What are some common ways to sort materials into groups? |  |
| 5. How does sorting materials into groups contribute to waste management practices? |  |















