Separation of Substances Chapter Notes | Eureka Plus Class 6: Book Solutions, Notes & Worksheets PDF Download
Introduction

Materials are all around us in various forms. These include things like air, soil, sand, water, gold, rocks, plastics, wood, ceramics, and oil.
Let's simplify and explain this idea further:
- Air, Soil, and Water: These are things we see and interact with every day. Air is what we breathe, soil is what plants grow in, and water is what we drink.
- Gold and Rocks: Gold is a shiny metal that is often used in jewelry, and rocks are hard substances found in nature like the stones you might see in a garden.
- Plastics: Plastics are materials that can be molded into different shapes. Items like water bottles and toys are often made of plastic.
- Wood: Wood comes from trees and is used to make furniture, houses, and paper. It's a natural material that is strong and versatile.
- Ceramics: Ceramics are materials like pottery and tiles that are made by shaping and firing clay. They are often used in kitchenware and home decor.
- Oil: Oil is a liquid that is used for fuel and making various products like plastic, gasoline, and lubricants. It comes from beneath the ground and is crucial for many aspects of modern life.
Pure Substances and Mixtures
Materials can be categorized broadly into two main groups: pure substances and mixtures. Understanding the difference between them is important in the study of chemistry.
Pure Substances
- A pure substance is entirely composed of only one type of substance.
- Examples of pure substances include sugar, oxygen gas, distilled water, gold, and alum crystals.
- In simpler terms, it's like having a bowl filled with only apples or a jar containing only sugar.
Mixtures
- A mixture consists of different things combined together.
- For instance, a salad made of raw cucumber, tomato, carrot, and sprouted mung beans is a mixture.
- Imagine a box filled with various items like toys, books, and clothes - that's a mixture.
Characteristics of Mixtures:
- The components of a mixture maintain their individual properties.
- All the components of a mixture can be separated from each other.
- Think of a bag of assorted candies where each candy tastes different; that's how components retain their properties in a mixture.
Reasons for separating substances from a mixture
Removing unwanted things
Sometimes, things we don't want get mixed with things we do want. For instance, when small stones get mixed with food grains, we need to separate them to make the grains safe to eat.
Separating useful substances
In nature, many things are mixed together. Sometimes, there are more than one useful thing in a mixture.
For example:
- Milk: From fresh milk, we can separate milk fat (ghee) and milk solids that contain proteins. This separation helps us get different useful products from milk.
- Petroleum: When we look at petroleum, we can extract petrol, diesel, kerosene, tar, and various useful chemicals (petrochemicals) from it. Each of these substances has its own purpose and use.
Separation of Solids
Handpicking

- In our daily lives, unwanted materials mixed in food grains, pulses, and other food items are often separated manually through handpicking.
Sieving

- Sieving is a method used to separate solid substances of different sizes. For instance, rice flour is sieved to remove large particles, including weevils (insects found in food grains).
- Sieves, typically made from wire mesh or metal plates with holes, vary in size based on the particles they are intended to separate.
- Heavy-duty sieves are also placed over drainage openings on roads to prevent solid waste from entering the drainage system during the rainy season.
Threshing

- When crops like paddy, wheat, or mustard are ready for harvest, the stalks are cut and dried in the sun.
- The dried stalks are then beaten to separate the grains from the stalks, a process known as threshing.
- Today, machines called threshers are commonly used for this purpose.
Winnowing
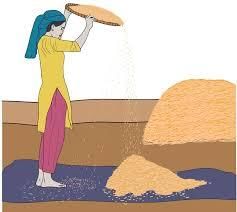
- Winnowing is an ancient technique for separating chaff (the outer covering of seeds) from grains and pulses.
- This simple and effective method involves dropping a mixture of light and heavy solid substances from a height, allowing the wind to carry away the lighter materials, thereby separating them from the heavier ones.
- Nowadays, winnowing is also performed using machines.
Separation of Insoluble Solids from Liquids
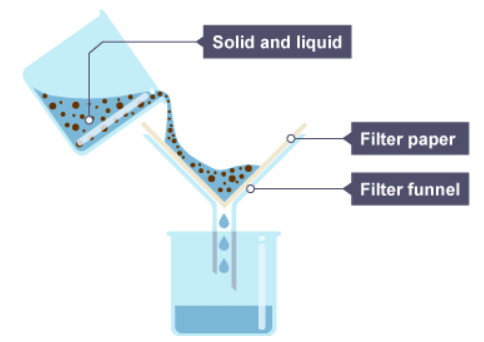
When we want to separate solid particles that don't dissolve in a liquid, we can use methods like sedimentation and decantation. These techniques help us to isolate the solid particles from the liquid they are mixed in.
Sedimentation
This process allows us to separate solid particles that do not dissolve in a liquid.
Here's how it works:
- Imagine you have a beaker with a mixture of sand and water. If you leave this beaker undisturbed for some time, the sand, which is heavier than water, will sink to the bottom of the beaker.
- The solid part that settles at the bottom of the beaker is called the sediment.
- Example: Think of a glass of muddy water. As the glass sits still, the mud or sand in the water starts to sink to the bottom.
 |
Download the notes
Chapter Notes: Separation of Substances
|
Download as PDF |
Decantation
Decantation involves carefully pouring off the liquid from one container to another without disturbing the sediment.
Here's how it's done:
- When you have a mixture where the solid has settled at the bottom (sediment), you slowly pour off the liquid on top into another container.
- This pouring process needs to be gentle to avoid mixing the settled solid back into the liquid.
- Example: Picture pouring water out of a glass with settled sand at the bottom, being careful not to disturb the sand.
Filtration
- Filtration is a technique used to separate insoluble particles from liquids using a porous medium known as a filter.
- A filter can be as simple as a fine piece of cloth used to strain water or a specialized paper employed in laboratories to filter liquids.
- The size of the filter’s pores is determined by the size of the solid particles that need to be separated.
- For instance, an air cooler’s filter is a fine plastic mesh, while a metal strainer for tea has larger pores.
Loading
- Sometimes, a liquid contains very small insoluble particles that remain suspended and take a long time to settle.
- Filtering such a mixture can be slow, as the tiny particles can clog the pores of the filter.
- To speed up the separation, a process called loading is used.
- For example, when alum is added to water containing fine soil particles, the alum binds to the suspended particles, making them heavier. As a result, the soil particles settle more quickly at the bottom. Alum, which can be found in grocery stores, facilitates this process.
Separation of Soluble Substances from a Liquid
Separating soluble substances from a liquid is like taking out some things that can dissolve in a liquid, leaving the liquid behind. One common way to do this is by using evaporation, which is turning a liquid into a gas by adding heat and letting the wind help.
Evaporation
This is when a liquid changes into a gas. It happens when the liquid gets heated up and the wind blows over it, taking some of the liquid away as gas.
- Using Heat and Wind: Heat and wind are helpers in the evaporation process. They make the liquid molecules move faster and leave the liquid as gas.
- Separating Solids from Water: Evaporation can help take out solid things that have dissolved in water. When you dry food in the sun, you are actually removing the water from it through evaporation.
- Obtaining Salt from Seawater: Seawater is mostly made of salt and water mixed together. To get salt from seawater, we use evaporation. The seawater is kept in shallow ponds where the water evaporates, leaving salt behind.
- Salt Pans: These are the shallow ponds where seawater is collected for evaporation. As the water disappears, salt is left behind on the ground of the salt pans.
Separating Immiscible Liquid
- Separating immiscible liquids involves the process of separating two liquids that do not mix together and instead form distinct layers.
- Examples of immiscible liquids include oil and water, which do not combine.
- To separate such liquids, a technique called decantation is employed.
Multiple Methods of Separation
In our daily lives, we often encounter mixtures of different substances. Separating these substances is important for various purposes such as cleaning, recycling, and purifying. Sometimes, using just one method for separation may not be enough, and we need to employ multiple methods to effectively isolate the components of a mixture.
- Handpicking: When we pick out pebbles from a mixture of pebbles and food grains by hand, we are using the method of handpicking to separate the larger particles.
- Winnowing: Imagine separating grains from chaff by tossing them in the air. The lighter chaff gets blown away by the wind while the heavier grains fall back down—it's like cleaning out a messy room by blowing away dust.
- Filtration: Filtering sand from water is akin to using a sieve or a strainer to separate solid particles from a liquid. It's like pouring a mixture through a fine mesh to catch the solid bits.
Complex Mixtures
- Oil, Sand, and Water Mixture: When dealing with a mixture of oil, sand, and water, a single method is not sufficient for separation due to the unique properties of these substances. Here's how we can separate them effectively:
- Allow Settling: Initially, we let the mixture sit undisturbed. This allows the oil, being less dense, to rise to the top of the water layer.
- Decantation: Carefully pouring off the oil without disturbing the water and sand layers is decantation. It's like slowly pouring out the top layer of a drink to avoid mixing the settled particles at the bottom.
- Sedimentation and Filtration: After removing the oil, we are left with a mixture of water and sand. Letting this mixture settle further allows the sand to sink to the bottom. By carefully pouring off the water (decantation) and then using a filter to capture the remaining sand particles, we can successfully separate all three components.
Unsaturated and Saturated Solutions
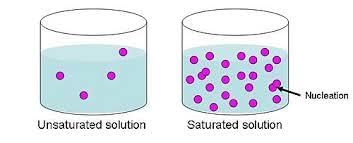
A solution is formed when a substance dissolves in a liquid. This solution consists of two main components: the solvent, which is the liquid that dissolves the substance, and the solute, which is the substance that dissolves in the solvent. For instance, in a sugar water solution, water is the solvent and sugar is the solute. If the solute is uniformly spread throughout the liquid, it's called a homogeneous mixture.
- Definition of Solvent and Solute: The solvent is the liquid part of a solution that dissolves another substance. The solute is the substance that dissolves in the solvent.
- Unsaturated Solutions: When a solution can still dissolve more solute, it's called an unsaturated solution. For example, if you keep adding salt to water and it keeps dissolving, it's unsaturated.
- Saturated Solutions: A saturated solution is formed when no more solute can be dissolved in the solvent. If you add salt to water and it stops dissolving, leaving some salt at the bottom, it's saturated.
- Solubility: Solubility is the maximum amount of solute that can dissolve in a given amount of solvent. Solubility increases with temperature. More salt can dissolve in hot water compared to cold water.
|
22 videos|80 docs|16 tests
|




























