Changes Around Us Chapter Notes | Eureka Plus Class 6: Book Solutions, Notes & Worksheets PDF Download
| Table of contents |

|
| Introduction |

|
| Desirable and Undesirable Changes |

|
| Fast and Slow Changes |

|
| Reversible and Irreversible Changes |

|
| Physical Changes |

|
| Chemical Change |

|
| A Change involves Energy |

|
Introduction
Every day, when you wake up, you see that it's bright outside because night has turned into day. This change from darkness to light is just one example of the many transformations that happen around us all the time. From flowers blooming in the morning to bees turning nectar into honey, nature is full of fascinating changes.
Key Points
- Day and Night Cycle: Night switches to day, bringing brightness in the morning.
- Flower Transformation: Flower buds bloom into beautiful flowers due to the gentle morning light.
- Bee Activity: Bees visit flowers, gather nectar, and transform it into honey.
- Fruit Formation: Flowers eventually transform into fruits.
- Universal Change: Every living thing experiences changes throughout its life.
- Transformation of Rocks: Even hard rocks change over time into tiny grains of sand.
Desirable and Undesirable Changes

- Some changes are beneficial and are considered desirable. For example, milk turning into curd, a seed germinating into a new plant, and bees converting nectar into honey are all desirable changes. Other examples include a flower developing into a fruit, the ripening of fruit, and water evaporating to form clouds.
- On the other hand, undesirable changes are those that are not beneficial and can even be harmful. While the ripening of a fruit is desirable, the rotting of fruit is an undesirable change. Disease-causing microorganisms lead to undesirable changes in the human body, making a person ill.
- Fire, which is used to cook food, is a desirable change, but it can also cause undesirable changes by burning and destroying things. Similarly, the force of flowing water can lead to both desirable and undesirable changes. It can be harnessed to produce electricity, but it can also cause floods, leading to property damage and loss of life.
- Understanding how a change occurs allows us to create desirable outcomes. For example, by understanding the conditions needed for maize seeds to germinate, we have learned to cultivate maize crops. We have also learned to make curd and cottage cheese from fresh milk.
- Similarly, we can prevent undesirable changes by knowing how they occur. For instance, we can protect an iron sheet from rusting by applying oil paint to it. Thus, understanding the process of change is very valuable.
Fast and Slow Changes
Fast and slow changes are two types of transformations that occur in the world around us. Fast changes happen quickly, while slow changes take a longer time to occur. Let's break down this concept into simpler terms and provide examples to understand it better.
Fast Changes
- Burning of paper is a fast change. Can you think of a few more fast changes?
- Other examples of fast changes include boiling water, popping a balloon, and breaking a glass.
Slow Changes
- The aging of the human body is a slow change that happens gradually over many years.
- A mango seedling growing into a tree is another example of a slow change that takes time to unfold.
- Natural processes like the formation of coal from wood, which occurs over millions of years, are extremely slow changes.
- Similarly, the formation of crude oil, soil, and certain types of rocks are also examples of slow changes that happen over vast periods of time.
Reversible and Irreversible Changes
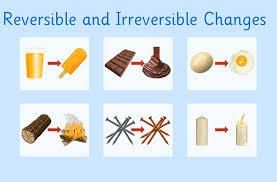
Reversible and irreversible changes are transformations that substances undergo. Reversible changes are those that can be undone, returning the substance to its original state, while irreversible changes are permanent alterations that cannot be reversed.
Reversible Changes
- When water is cooled to 0 degrees Celsius, it becomes ice. This change is reversible, meaning ice can turn back into water when it warms up.
- Just like water freezing into ice and then melting back into water, changes like these that can be reversed are called reversible changes.
- An example of a reversible change is knitting a cap with wool. If the cap is unraveled, the wool can be collected and reused, showing how the change can be undone.
- Another example is bees turning nectar into honey, which is also a reversible change as honey can be changed back into a liquid state.
- Metals melting and solidifying at specific temperatures are also reversible changes, allowing them to be molded into different shapes.
Irreversible Changes
- When a potato is boiled, it becomes soft. This change cannot be reversed, making it an irreversible change.
- Similarly, burning paper turns it into carbon, which is irreversible. These kinds of changes are called irreversible changes.
- Irreversible changes, unlike reversible ones, are usually chemical changes and cannot be undone.
- Examples of irreversible changes include making dough from wheat flour, burning wood, rusting of an iron nail, and chopping vegetables.
 |
Download the notes
Chapter Notes: Changes Around Us
|
Download as PDF |
Physical Changes
Every material possesses certain characteristics related to its appearance, size, temperature, and state (solid, liquid, or gas), which are known as physical properties. When a change only impacts these physical traits without altering the fundamental nature of the material, it is termed a physical change. These alterations do not create new substances but rather modify the existing material in a reversible manner.
Key Points
- Physical changes involve transformations that affect only the external properties of a substance without forming a new material.
- Examples of physical changes include folding a piece of paper to make an airplane, evaporation of water, and freezing water to form ice.
- During physical changes, only certain aspects like the state (solid, liquid, gas), temperature, and volume of the substance are altered.
- Physical changes are reversible processes, meaning the original material can often be restored to its initial state.
- Instances of physical changes include mixing sand with water, cutting wood, and breaking glass, where the material's composition remains unchanged.
- Salt dissolving in water to create a solution and the gentle heating of wax are additional examples of physical changes.
- Physical changes do not lead to the creation of new substances, and the properties of the original materials involved are retained.
- Separation methods such as evaporation can be used to recover the individual components of a physically changed mixture (like salt and water).
Chemical Change
When you heat sugar gently, it first melts. The melted sugar still tastes like sugar and dissolves in water, retaining its original properties, which shows it's a physical change. However, if you keep heating it, the sugar will eventually burn and turn into a black substance called carbon. This new substance is different from sugar and indicates a chemical change. Chemical changes are transformations where new substances with different properties are formed.
Key Points
- Physical Change: Heating sugar until it melts is a physical change, as the melted sugar retains its sugar properties like taste and solubility in water.
- Chemical Change: Continuing to heat sugar causes it to burn and transform into carbon, a substance with different properties than sugar. This change is a chemical change.
- Examples of Chemical Changes:
- Rusting of iron: When iron rusts, it undergoes a chemical change, forming a different substance with properties different from iron.
- Formation of curd from milk: The process of curdling milk to form curd involves a chemical change where new substances are created.
- Cooking of food: The transformation of raw ingredients into cooked food involves various chemical changes that alter the properties of the ingredients.
- Ripening of fruits: The ripening of fruits is a chemical change where the fruit undergoes transformations in its composition and properties.
- Irreversibility of Chemical Changes: Most chemical changes, once they occur, are irreversible, meaning it is difficult or impossible to revert the substances back to their original form.
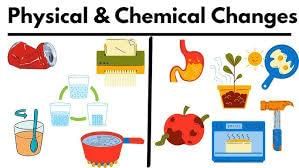
Differences between physical and chemical changes
When we talk about changes in matter, we can broadly categorize them into two types: physical changes and chemical changes. Understanding the differences between these two types of changes is essential in science.
- Physical changes:
- Physical changes are like when you change the way something looks or feels, but it's still the same thing at the end.
- These changes are usually reversible, meaning you can often change things back to how they were before.
- During a physical change, no new substances are created. The stuff you started with is the same as what you end up with.
- The properties of the material undergoing the change remain the same. It's like changing the shape of a piece of clay without making it into something entirely different.
- Chemical changes:
- Chemical changes are more like when you mix things together and they form something entirely new.
- These changes are usually irreversible, meaning you can't easily change things back to how they were before.
- When a chemical change happens, new substances are created. These new substances have different properties from the original substances.
- It's like when you bake a cake - you mix ingredients together, heat them up, and what you get is a cake, which is completely different from the flour, eggs, and sugar you started with.
Understanding physical and chemical changes helps us make sense of the world around us. Whether it's freezing water into ice (a physical change) or burning wood to ash (a chemical change), these concepts are fundamental in explaining how matter behaves and transforms.
A Change involves Energy
When we talk about changes happening around us, energy plays a crucial role. Energy is like the power that causes things to happen. For instance, think about how sunlight helps a sunflower bloom or how heat melts wax. These examples show how energy is involved in different processes that lead to changes.
- Changes and Energy:
- Energy is what we use when changes occur. It's like the force that makes things happen.
- Example: When sunlight hits a sunflower bud, it uses energy from the sunlight to bloom.
- Forms of Energy:
- Heat is a form of energy that we often encounter. When you burn wood, it undergoes a chemical change and releases heat and light energy.
- Light itself is a form of energy as well.
- States of Matter:
- By adding or removing heat energy, we can change the states of matter. For example, think about how ice melts into water when heat is applied to it.
- Overall Concept:
- Whenever a change happens, energy is always involved. It's like the invisible force driving transformations all around us.
|
22 videos|80 docs|16 tests
|























