Instrumentation Methods: Spectrophotometer | Zoology Optional Notes for UPSC PDF Download
Introduction to Spectrophotometers
- A spectrophotometer stands as an indispensable tool within the realm of spectroscopy, dedicated to the quantitative measurement of a material's reflection or transmission properties contingent on its wavelength.
- These instruments, often referred to as photometers or spectrophotometers, possess the capability to gauge the intensity of light across a spectrum of wavelengths.
- Traditionally, spectrophotometry focuses on the analysis of ultraviolet, visible, and infrared radiation. However, contemporary spectrophotometers extend their reach to encompass a wide electromagnetic spectrum, covering x-ray, ultraviolet, visible, infrared, and microwave wavelengths.
- The fundamental purpose of a spectrophotometer is to gauge the quantity of light absorbed by a given sample.
- This is achieved by channeling a light beam through the sample, followed by the measurement of light intensity after its interaction with the material.
- The information gleaned from this process aids in deducing various attributes of the sample, such as its concentration or its propensity to absorb specific wavelengths of light.
- Spectrophotometer techniques find practical utility in measuring solute concentrations within solutions.
- This is accomplished by assessing the quantity of light absorbed by a solution contained within a cuvette, placed within the spectrophotometer.
- The precise analysis of light absorption patterns facilitates an accurate determination of the solute's concentration within the solution.
- An iconic instrument in the world of spectrophotometry, the Beckman DU spectrophotometer, holds historical significance.
- In 1940, scientist Arnold J. Beckman and his colleagues at the National Technologies Laboratory (NTL) pioneered this remarkable invention.
- The Beckman DU spectrophotometer significantly contributed to the progress of spectrophotometry and its wide-ranging applications across diverse scientific domains.
Key Components
- A spectrophotometer primarily comprises two primary components: the spectrometer and the photometer.
- The spectrometer emits light at specific wavelengths, allowing it to pass through the sample.
- Simultaneously, the photometer detects the quantity of light absorbed by the solution.
- The critical analytical process involves comparing the intensity of the incident light with the intensity of light that has traversed the sample.
- In summation, the spectrophotometer is an incredibly versatile laboratory instrument dedicated to assessing light absorption by substances.
- Its applications extend across a multitude of scientific disciplines, enabling researchers to gain a quantitative understanding of material properties and behavior with exceptional precision.
In essence, spectrophotometers play a pivotal role in understanding the interaction between materials and light, allowing for quantitative analysis with broad applications in scientific research and experimentation.
Illuminating the Principle of Spectrophotometry
- Spectrophotometry, a scientific methodology, quantifies light intensity concerning its wavelength.
- This approach hinges on the foundational principles of photometry, which examine the interplay between light and substances.
- Spectrophotometers, as instruments, leverage these principles to analyze a compound's absorption spectrum, offering critical insights into its light absorption across diverse wavelengths.
- Central to a spectrophotometer is a mechanism, typically a prism or grating, dispersing incident light into its constituent wavelengths.
- Precise selection mechanisms allow specific wavelengths to be directed at a test solution, with an impressively narrow range of 1 to 2nm.
- Spectrophotometry revolves around the photometric principle, manifesting when an initial light intensity (I0) interacts with a solution.
- During this interaction, light can be reflected (Ir), absorbed (Ia), and transmitted (It), adhering to the mathematical relationship: I0 = Ir + Ia + It.
- In spectrophotometric measurements, the goal is to ascertain Ia, the absorbed light. Eradicating Ir is achieved by employing cells with identical properties, ensuring uniform reflection. Consequently, the measurement of I0 and It provides the necessary information to deduce Ia.
- Two fundamental laws govern the relationship between absorbed light and the concentration of the absorbing substance in a solution: Beer's Law and Lambert's Law.
- Beer's Law asserts that light absorbed by a solution is directly proportional to its solute concentration, denoted mathematically as log10(It/I0) = asc.
- Lambert's Law contends that the absorbed light is directly proportional to the path length (b) or thickness of the solution, represented as A = log10(It/I0) = asb.
- Combining both Beer's and Lambert's Laws results in the Beer-Lambert Law, depicted as log10(It/I0) = asbc.
- If the path length (b) remains constant, the equation simplifies to log10(It/I0) = asc.
- The absorbency index (as) is further defined as as = clA, with c representing the concentration of the absorbing material in gm/liter and l signifying the distance traveled by light in the solution in cm.
- Spectrophotometers function based on the amalgamation of Beer-Lambert Laws, stipulating that the absorbance of a colored solution is directly proportional to its concentration and the path length of light through it.
- This relationship is succinctly conveyed as A∝cl or, with the absorption coefficient ε, as A = εcl.
In summary, spectrophotometry, rooted in photometric principles, is a robust technique that delves into the interaction between light and matter, rendering valuable insights into the characteristics of diverse compounds.
Diverse Spectrum of Spectrophotometers
Introduction to Types of Spectrophotometers
Spectrophotometers encompass a multitude of types, each tailored to different wavelength ranges, light configurations, and applications. These versatile instruments play an essential role in various scientific and analytical endeavors. Here are some common types of spectrophotometers:
UV-Visible Spectrophotometer
- Utilizes light in the ultraviolet (UV) and visible range (UV: 185-400 nm, Visible: 400-700 nm) of the electromagnetic spectrum.
- Commonly used for analyzing colored compounds and determining their concentration in solutions.
IR Spectrophotometer (Infrared)
- Operates within the infrared range (700-1500 nm) of the electromagnetic spectrum.
- Analyzes substances that absorb infrared light, offering insights into functional groups and molecular structures.
Single Beam Spectrophotometer
- Operates within specific wavelength ranges (e.g., 325-1000 nm) using a single beam of light.
- Measures absorbance sequentially for the test solution and a reference solution in the same cuvette.
Double Beam Spectrophotometer
- Splits light from a monochromator into two beams: one serves as a reference, while the other passes through the sample for measurement.
- Facilitates simultaneous measurement of the reference and sample, enhancing stability and user-friendliness.
Visible Light Spectrophotometer
- Utilizes visible light from a tungsten lamp, often employed in routine laboratory practices.
- Available in portable and bench-top models.
UV-Vis Spectrophotometer (Ultraviolet-Visible)
- Versatile instruments measuring a wide range of wavelengths, often up to 1100 wavelengths.
- Combines UV and visible light, offering features such as scanning functions, user interfaces, integral printers, and multiple cell settings.
Near-Infrared Spectrophotometer (NIR)
- Measures a sample's response to infrared light.
- Useful for non-invasive analysis, providing quantitative results with minimal sample preparation.
Nuclear Magnetic Resonance (NMR) Spectroscopy
- Determines the structure and dynamics of organic compounds.
- Offers detailed insights into the entire molecule's structure and organic reactions.
Mercury Spectrophotometer/Analyzer
- Specifically designed to measure mercury levels in water, ensuring precise detection and analysis.
Fluorometers
- Measure the fluorescence emitted by a sample when exposed to a specific wavelength of light.
- Commonly used in fields such as biochemistry and environmental analysis.
Atomic Absorption Spectrophotometer
- Employs a flame to vaporize a sample, causing it to dissociate into ions.
- Determines sample concentration by measuring changes in light intensity.
- Widely used in toxicology, environmental testing, and quality control laboratories.

These diverse types of spectrophotometers cater to specific applications and requirements within the expansive realm of spectroscopy. Researchers and analysts select the most suitable type based on the nature of the samples and the desired analytical outcomes.
Components of a Spectrophotometer
A spectrophotometer, an indispensable tool in scientific laboratories, relies on the principles of light absorption and emission to provide precise measurements of light intensity across varying wavelengths. The device's intricate design comprises several key components, each with a specific function in the analytical process. Let's delve into the fundamental parts of a spectrophotometer and explore their roles:
1. Radiant Energy Source
- The foundational element of any spectrophotometer is its light source.
- Radiant energy sources are materials capable of reaching high-energy states through electrical heating or high-voltage electric discharge.
- Common light sources include tungsten lamps for visible light, hydrogen, and deuterium lamps for ultraviolet radiation, and Nernst filaments or globar for infrared radiation.
2. Monochromator
- A pivotal component responsible for isolating specific wavelengths from the polychromatic radiation emitted by the light source.
- Resolves and narrows down individual wavelengths into specific bands, ensuring that only the desired wavelength interacts with the sample.
3. Prisms
- Prisms play a crucial role in dispersing polychromatic light into its constituent wavelengths.
- Their ability to reflect different wavelengths to varying extents results in the dispersion process.
- Two primary prism types used in commercial spectrophotometers are the 600 cornu quartz prism and the 300 Littrow Prism.
4. Grating
- Particularly prevalent in spectrophotometers operating in the ultraviolet, visible, and infrared regions.
- Further refines the process of wavelength isolation.
5. Sample Holder (Cuvettes)
- Specialized containers designed to hold the samples under analysis, typically solutions or glasses.
- Cuvette materials vary based on the region of study, with options such as ordinary glass or quartz.
6. Photosensitive Detector and Readout System
- The core of a spectrophotometer's measurement capability resides in its detector.
- Most detectors operate on the photoelectric effect principle, with generated current directly proportional to light intensity.
- Electronic signals, representative of transmitted light, are converted into interpretable forms using amplifiers, ammeters, and potentiometric recorders.
7. Beam Splitter and Mirror (Double-Beam Spectrophotometers)
- Exclusive to double-beam spectrophotometers.
- The beam splitter divides the incoming light beam into two separate beams.
- Mirrors then guide these beams in the correct direction for further analysis.
8. Measuring Device
- The electric current from the detector is routed to a measuring device, often a galvanometer.
- Readings on this device are directly proportional to light intensity, providing quantitative data on the sample's light absorption or transmission properties.

Each of these components in a spectrophotometer contributes to its ability to precisely analyze the absorption and emission of light, making it an invaluable instrument in scientific research and analysis.
Here's a tabular representation of the components of a spectrophotometer and their descriptions: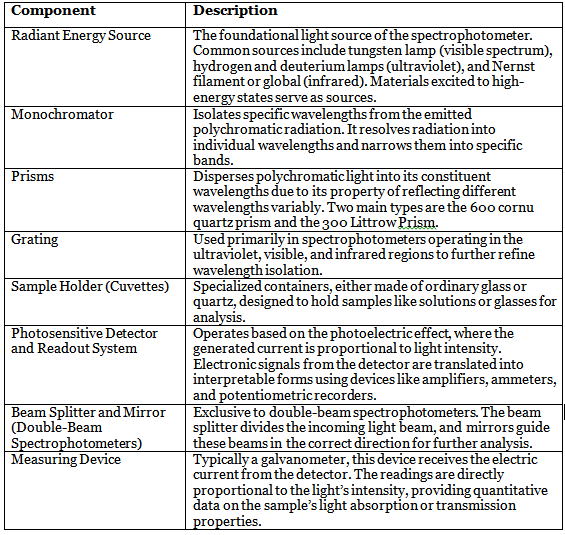
How a Spectrophotometer Works: The Working Mechanism
A spectrophotometer, an essential analytical instrument, measures light absorption by a sample at various wavelengths. This explanation details the working mechanism of a spectrophotometer:
- Calibration for Accuracy: Prior to use, calibration with standard solutions of known concentration ensures precision and establishes a reference point for comparisons.
- Selection of Wavelength: epending on the analyzed solution, the spectrophotometer allows precise selection of the desired wavelength for testing.
- Passage of Light: Light from the source undergoes a series of components within the spectrophotometer. It initially encounters a diffraction grating, prism, and mirror, contributing to light manipulation and direction.
- Role of Diffraction Grating and Prism: The diffraction grating disperses incoming light into multiple wavelengths. The prism further dissects the light into its constituent wavelengths, enabling the isolation of the required wavelength for analysis.
- Interaction with Sample in Cuvette: Monochromatic light, as selected, traverses a cuvette containing the sample solution (either the test or standard solution). Interaction with the solution results in light absorption, reflection, and transmission.
- Photodetector System: After passing through the cuvette, the transmitted light is captured by a photodetector system. This system gauges the intensity of the transmitted light and transforms it into electrical signals.
- Galvanometer and Absorbance Measurement: Electrical signals from the photodetector system are conveyed to a galvanometer. The galvanometer quantifies the received signal, which corresponds to the absorbance of the solution. Absorbance signifies the logarithmic measure of absorbed light.
- Concentration Calculation: Solution concentration is derived from absorbance utilizing the Beer-Lambert law: C = A/(εL), where C represents solution concentration, A is absorbance, ε signifies the molar extinction coefficient (a substance-specific constant), and L is the path length the light travels through in the cuvette.
- Double Beam Spectrophotometer: In certain spectrophotometers, like double beam spectrophotometers, monochromatic light is split into two beams. One beam passes through a standard solution, while the other passes through the test solution, enabling simultaneous analysis and comparison of both samples.
Following these steps, a spectrophotometer offers quantitative insights into the concentration or absorption properties of the examined sample. This versatile tool finds applications across diverse fields including chemistry, biochemistry, environmental science, and pharmaceutical analysis.
Comparison of Single Beam vs. Double Beam Spectrophotometers
Spectrophotometers, essential instruments in scientific analysis, exist in two primary categories: single beam and double beam spectrophotometers. Let's explore the characteristics, functionalities, and differences between these two types:
1. Single Beam Spectrophotometers:
- Light Beam: Single beam spectrophotometers employ a single light beam, typically in the visible or UV range.
- Measurement Method: The light beam passes through the sample within a cuvette, and the intensity is measured both before and after interacting with the sample.
- Principle: They apply Beer-Lambert's Law, which correlates light absorbance with the analyte's concentration, enabling concentration calculations.
- Size and Dynamic Range: Single beam spectrophotometers are generally compact and possess a broader dynamic range.
- Applications: Suited for routine measurements and situations where high precision or automation is not a critical requirement.
- Limitation: May not fully account for background absorption by the solvent, potentially impacting measurement accuracy.
2. Double Beam Spectrophotometers:
- Light Beam: Double beam spectrophotometers work similarly to single beam instruments but feature a distinct attribute.
- Measurement Method: The initial light source is divided into two separate beams. One beam traverses the sample, while the other travels through either a reference solution or the solvent alone.
- Absorbance Calculation: Measurements rely on the ratio of the two light beams, representing the sample's absorbance.
- Advantages: Double beam spectrophotometers provide higher levels of automation, enabling more precise and accurate measurements. Simultaneous measurement of sample and reference facilitates compensation for background absorption due to the solvent and other factors, enhancing measurement reliability and accuracy.
- Applications: Ideal for scenarios demanding high accuracy or when dealing with complex samples.
Summary: Single beam spectrophotometers are characterized by their compact size and broad dynamic range, making them suitable for routine measurements. In contrast, double beam spectrophotometers offer improved precision, automation, and the capability to correct for background absorption, rendering them ideal for applications requiring high accuracy or involving intricate sample compositions. The choice between these spectrophotometer types depends on the specific needs and the level of accuracy essential to the analysis.
Measuring Absorption With a Spectrophotometer
To measure the absorption of a sample using a spectrophotometer, you should follow these steps:
Set up the Spectrophotometer:
- Turn on the spectrophotometer and allow it to warm up according to the manufacturer's recommendations.
- Ensure proper calibration as per the manufacturer's instructions to establish an accurate baseline for measurements.
Prepare the Sample:
- Prepare a solution of the sample to be analyzed, ensuring complete dissolution for homogeneity.
- If necessary, dilute the sample to bring its concentration within the spectrophotometer's linear range.
Select the Appropriate Wavelength:
- Determine the wavelength of light that corresponds to the maximum absorption of your sample. This information can be obtained from literature sources or preliminary tests.
- Set the spectrophotometer to this specific wavelength.
Blank the Spectrophotometer:
- Place a blank sample (containing all components except the substance being measured) in the cuvette holder. This blank serves as a reference for baseline correction.
Insert the Sample Cuvette:
- Remove the blank and insert the cuvette containing your sample into the spectrophotometer.
- Ensure proper alignment with the light beam and check for the absence of air bubbles or contaminants that could affect measurements.
Measure the Absorbance:
- Close the spectrophotometer's lid and initiate the measurement.
- The spectrophotometer will emit light at the selected wavelength through the sample cuvette.
- It measures the intensity of light transmitted through the sample and compares it to the intensity of the reference blank.
- The result displayed is the absorbance (A) of the sample at the selected wavelength.
Record the Data:
- Take note of the absorbance value displayed on the spectrophotometer, indicating the extent of light absorbed by the sample at the given wavelength.
Remember to ensure the consistency of units (molar extinction coefficient, concentration, and path length) during calculations to prevent errors in the final result. By following these steps and applying Beer-Lambert's Law, you can effectively measure the absorption of a sample using a spectrophotometer.
Limitations of Spectrophotometer
Spectrophotometers, while versatile instruments, have limitations that affect their effectiveness and the types of measurements they can accurately perform. Here are some key limitations:
Inability to Measure Uncolored Compounds:
- Spectrophotometers cannot measure chemicals that lack color or do not absorb UV light.
- Compounds without inherent color or UV absorption can be analyzed by introducing reagents that generate colored products for measurement.
Optimal Range for Absorbance Readings:
- Spectrophotometers work best within a specific range of absorbance readings, typically between 0 and 1.
- Samples with absorbance readings beyond this range may require appropriate dilution and application of a dilution factor for accurate concentration determination.
Understanding these limitations is crucial for ensuring the accurate and reliable application of spectrophotometry in various scientific and analytical contexts.
Preventive Measures for Working with a Spectrophotometer
When operating a spectrophotometer, it is essential to take specific preventive measures to ensure the accuracy and reliability of measurements. Here are key preventive measures to consider:
Warm-up Time:
- Turn on the spectrophotometer approximately 10 to 15 minutes before usage. This allows the instrument to stabilize and ensures consistent performance.
Calibration:
- Calibrate the spectrophotometer before each use. Calibration involves employing standard solutions of known concentrations to establish a baseline and ensure accurate measurements. This process corrects for variations or drift in the instrument's performance.
Selecting Wavelength:
- Choose the appropriate wavelength for your measurement. Select the wavelength at which the sample absorbs light most strongly. This ensures absorbance readings are within the optimal range for accurate quantification.
Sample Integrity:
- Ensure that the sample used does not contain any substances that can dissociate, react, or change during the measurement. Such reactions can interfere with the accuracy of results. Handle and store samples properly to maintain their stability.
Concentration Range:
- Ensure the sample concentration falls within the acceptable range for accurate measurements. Concentrations that are too high or too low can result in non-linear responses or detector saturation. Dilution or concentration adjustments may be needed to bring the sample within the optimal range.
By following these preventive measures, you can enhance the reliability and accuracy of your spectrophotometric measurements. Additionally, consulting the instrument's user manual and adhering to the manufacturer's guidelines is advisable for proper operation and maintenance.
What a Spectrophotometer Measures
A spectrophotometer is an analytical instrument used to measure how a substance interacts with light across a range of wavelengths. It quantifies the amount of light absorbed or transmitted by a substance, depending on its mode of operation and the type of analysis performed. The primary measurements made by a spectrophotometer include:
Absorbance (Optical Density):
- Spectrophotometers measure the absorbance of a sample at specific wavelengths. Absorbance (often denoted as "A") quantifies the amount of light absorbed by a substance at a particular wavelength. It is commonly used in quantitative chemical analyses to determine the concentration of a solute using the Beer-Lambert Law.
Transmittance:
- Transmittance (often denoted as "T") measures the fraction of incident light that passes through a sample. It is the complement of absorbance, calculated as T = 100% – A%. Transmittance assesses the transparency of a material or the extent to which light can pass through it.
Reflectance:
- In certain applications, particularly for solid samples, spectrophotometers measure the amount of light reflected by a material. Reflectance measurements are valuable for characterizing the optical properties of materials, including the color of pigments, coatings, and surfaces.
Spectral Data:
- Spectrophotometers provide spectral data, which is a plot of absorbance, transmittance, or reflectance of a substance as a function of wavelength. These spectra reveal how the sample interacts with light at different wavelengths and are valuable for identifying compounds and studying their electronic or molecular structures.
Color Measurement:
- Spectrophotometers are used for colorimetry to assess the color of a substance by measuring the absorption or reflectance of specific wavelengths associated with color perception. This is crucial in industries like textiles, food, and cosmetics.
Kinetic Measurements:
- Some spectrophotometers can perform kinetic measurements by monitoring changes in absorbance or transmittance over time. This capability is employed in enzyme assays, chemical reaction monitoring, and other time-dependent analyses.
In summary, a spectrophotometer is a versatile instrument that measures the interaction between light and a sample. It provides quantitative data on absorbance, transmittance, and reflectance, and offers spectral data for various scientific, chemical, and industrial applications, ranging from quantifying substance concentrations to assessing the optical properties and colors of materials.
Difference Between a Spectrometer and a Spectrophotometer
A spectrometer and a spectrophotometer are two distinct scientific instruments that deal with the properties of electromagnetic radiation and the characteristics of substances. They serve different purposes and operate in varying modes. Here are the key differences between them:Spectrometer:
- A spectrometer is used for the qualitative and quantitative analysis of light emitted or absorbed by a substance.
- It is versatile and employed in diverse scientific disciplines, including chemistry, physics, and astronomy.
- In astronomy, spectrometers determine the temperature of celestial bodies by analyzing emitted light wavelengths and measure Doppler shifts to determine the speed of celestial objects.
- In scientific research and laboratories, spectrometers identify the elemental composition of substances by analyzing characteristic spectral lines produced when atoms or molecules interact with light.
- They are crucial in biomedical research for detecting toxins, contaminants, and diseases by analyzing spectral signatures in biological samples like blood or urine.
- A spectrophotometer specifically measures the intensity of electromagnetic radiation at different wavelengths, usually in the ultraviolet, visible, and near-infrared regions.
- Its primary function is to determine the absorbance, transmittance, or reflectance of light in a given sample.
- Spectrophotometers excel in quantifying the absorbance of light by a substance, widely used in chemistry and biochemistry laboratories for concentration determination using the Beer-Lambert Law.
- They assess the transparency of solids and the reflectance of solutions, essential in characterizing materials used in industries such as optics, coatings, and electronics.
- Spectrophotometers can be double-beam or basic instruments, offering different measurement methods and precision levels.
In conclusion, while both spectrometers and spectrophotometers deal with the interaction of light and matter, they have distinct purposes and modes of operation. Spectrometers are versatile instruments for analyzing emitted or absorbed light, determining composition, and performing temperature and speed measurements. Spectrophotometers are specialized tools that focus on measuring light absorbance, transparency, and reflectance, with applications in chemistry, biochemistry, and materials characterization. Each instrument plays a unique and crucial role in advancing scientific knowledge and practical applications across various fields.
Difference between a Colorimeter and a Spectrophotometer
Colorimeters and spectrophotometers are both analytical instruments used to assess the color and light-absorbing characteristics of substances. However, they differ in various aspects, including their functionality, spectral range, and applications.1. Functionality:
Colorimeter:
- A colorimeter measures the absorbance of a specific color in a sample.
- It uses a set of predefined color filters or LEDs to emit specific colors of light.
- Users select a filter corresponding to the color they want to measure, and the colorimeter quantifies the absorbance of that specific color.
- Colorimeters are suitable for simple color analysis tasks.
- A spectrophotometer measures the transmittance or reflectance of light across a range of wavelengths, providing a full spectrum of the sample's interaction with light.
- It operates across a broader spectral range, including the ultraviolet (UV), visible, and often infrared (IR) regions of the electromagnetic spectrum.
- Spectrophotometers are capable of performing quantitative analysis based on the entire spectrum, offering comprehensive data for various analytical needs.
- They are used in a wide range of scientific and industrial applications.
Colorimeter:
- Colorimeters are limited to the visible part of the electromagnetic spectrum, covering wavelengths from approximately 400 nm (violet) to 750 nm (red).
- They are specifically designed for analyzing the absorption of visible light by samples.
- Spectrophotometers operate across a wider spectral range, including UV, visible, and sometimes IR regions.
- This extended range allows for the characterization of samples using a more comprehensive spectrum of electromagnetic radiation.
3. Applications:
Colorimeter:
- Colorimeters are typically used for routine color analysis, such as in quality control for products like paints, textiles, and food, where a full spectrum analysis is not necessary.
- They are suitable for applications where a specific color is of primary interest.
Spectrophotometer:
- Spectrophotometers are employed in a wide range of applications, including chemical analysis, environmental monitoring, biochemical assays, and more.
- They are essential when precise quantitative analysis or the examination of samples across a broad range of wavelengths is required.
- Spectrophotometers are versatile instruments with applications in various scientific and industrial disciplines.
4. Cost:
Colorimeter:
- Colorimeters are generally less expensive than spectrophotometers due to their limited functionality and narrower spectral range.
Spectrophotometer:
- Spectrophotometers tend to be more expensive because of their advanced capabilities, extended spectral range, and complex optical systems.
In summary, colorimeters and spectrophotometers are valuable tools for color and light-absorption analysis, but they are suitable for different purposes. Colorimeters are best suited for simple color analysis tasks within the visible spectrum, whereas spectrophotometers offer a broader analytical capability and are indispensable for more complex quantitative analyses in various scientific and industrial fields. The choice between the two instruments depends on specific analytical requirements and budget considerations.

Note: This table provides a structured comparison between colorimeters and spectrophotometers, highlighting their differences in terms of functionality, spectral range, cost, and primary applications, aiding in the selection of the appropriate instrument for specific analytical needs.
|
181 videos|346 docs
|

|
Explore Courses for UPSC exam
|

|


















