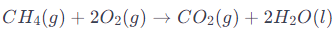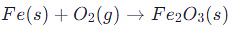Oxidation-Reduction Reactions | Zoology Optional Notes for UPSC PDF Download
| Table of contents |

|
| Biological Oxidation-Reduction Reactions |

|
| Oxidation State: The Bookkeeper's Tool |

|
| Unveiling the Oxidation States |

|
| Electrifying Biochemical Redox |

|
Biological Oxidation-Reduction Reactions
In the intricate ballet of cellular processes, biological oxidation-reduction reactions, often referred to as "redox" reactions, play a fundamental role in orchestrating the flow of electrons. This complex choreography involves the simultaneous occurrence of oxidation and reduction, where electrons are exchanged among species, driving essential metabolic pathways.
Defining Redox Reactions
Redox reactions encompass a vast array of chemical transformations, characterized by the exchange of electrons. Two key players in this dance are oxidation and reduction:
- Oxidation: Involves the loss of electrons by a species during a chemical reaction.
- Reduction: Signifies the gain of electrons by a species in the course of a reaction.
The conservation of electrons ensures that for every oxidation, there is a corresponding reduction – a harmonious interplay reminiscent of the yin-yang principle.
Oxidation State: The Bookkeeper's Tool
To unravel the secrets of redox reactions, chemists employ the concept of oxidation state or oxidation number. This bookkeeping device aids in classifying and understanding chemical transformations. Here's a brief guide:
- Elemental Form: The oxidation state of an atom in its elemental form is zero.
- Monatomic Ions: The oxidation state of an atom in a monatomic ionic form equals the charge on the ion.
- Sum Rule: The sum of all oxidation states in a molecule or ion is equal to the net charge.
A hierarchy of oxidation states based on valences and electronegativity guides the assignment of oxidation states for individual elements.
Rules for Assigning Oxidation States
- Elemental Form: Zero oxidation state for atoms in their elemental form.
- Monatomic Ions: Oxidation state equals the charge on the ion.
- Sum Rule: Sum of oxidation states in a molecule equals the net charge.
- Hierarchy: Follows a hierarchy based on valences and electronegativity.
Examples of Redox Reactions
Combustion of Methane:

- Involves the oxidation of carbon in methane and reduction of oxygen to water.
Rusting of Iron:

- Represents the oxidation of iron and reduction of oxygen.
Formation of Salt:
- 2Na(s)+Cl2(g)→2NaCl(s)
- Involves the combination of elemental sodium and chlorine to form an ionic compound.
Beyond Uncombined Elements: Identifying Redox Reactions
Even without uncombined elements, redox reactions persist, as illustrated by:
8H+(aq) + 2NO3−(aq) + 3Sn2+(aq) → 4H2O(l) + 2NO(g) + 3Sn4+(aq)
16H+(aq) + 3C2H5OH(aq) + 2Cr2O72−(aq) → 11H2O(l) + 3CH3CO2H(aq) + 4Cr3+(aq)
These reactions showcase the dynamic interplay between oxidation and reduction, exemplifying the versatility of redox chemistry.
Conclusion: The Elegance of Electron Flow
In the grand tapestry of biological systems, redox reactions emerge as pivotal players, facilitating energy transfer, metabolic pathways, and maintaining cellular equilibrium. The assignment of oxidation states serves as a guide, allowing chemists to discern the intricate steps of this electron dance and unravel the mysteries of life's fundamental processes.
Unveiling the Oxidation States
In the intricate world of biochemical reactions, the oxidation states of carbon atoms and oxygen compounds unveil a mesmerizing dance of electrons. Let's explore this symphony, where changes in oxidation numbers orchestrate the intricate steps of reduction and oxidation.
Oxidation States of Carbon in One-Carbon Molecules
In the realm of one-carbon molecules comprising carbon (C), hydrogen (H), and oxygen (O), the oxidation states of carbon atoms undergo a harmonious transformation. Note the elegant pattern: the oxidation number changes in steps of two, synchronized with the addition or loss of two electrons. This dance is intricately tied to the number of bonds the carbon atom forms with oxygen, a key player in this biochemical ballet.
Example: Reduction of Formaldehyde to Methanol
Let's dissect the reduction of formaldehyde to methanol as a representative vignette. The unbalanced reduction half reaction unfolds:
CH2O+2e−→ CH3OH
To bring balance to this equation, we step into the realm of acidic conditions. Adding protons (H+) on both sides balances hydrogen atoms and charge, resulting in the refined and balanced reduction half reaction:
CH2O+2H++2e− → CH3OH
Reactive Oxygen Species (ROS): The Perilous Intermediates
As we delve into the realm of oxygen compounds, a series of biological significance unfolds. The grand finale of oxidative phosphorylation culminates in the reduction of molecular oxygen to water. Yet, lurking in the shadows are reactive oxygen species (ROS), partially reduced oxygen intermediates. These elusive species, with oxidation states intermediate between molecular oxygen and water, pose a threat to cellular integrity.
Summary of ROS and Their Oxidation States (Oxygen Atom[s])
- Superoxide Radical (O2−): -1
- Hydrogen Peroxide (H_2O_2): -1
- Hydroxyl Radical (OH−): -2
- Singlet Oxygen (1O2): 0
Caution: Danger Lurks in the Reduction of O2
The reduction of molecular oxygen in cells engaged in oxidative metabolism is a tightly choreographed ballet. However, this cautionary note warns us of the peril associated with the production of reactive oxygen species. The reactivity of ROS poses a threat to cellular components, causing damage to membrane lipids, proteins, and nucleic acids.
In the delicate interplay of biochemical reactions, understanding oxidation states becomes a key to deciphering the language of electrons. It allows us to compose balanced half reactions, providing insights into the nuanced processes that sustain life and, occasionally, harbor danger in their intricacies.
Electrifying Biochemical Redox
In the captivating realm of biochemical redox reactions, the language of charge, Faraday, and electron carriers weaves a fascinating tale of energy transfer and electron flow. Let's unravel the electrifying details.
Charge, Faraday, and Current: Measuring the Dance of Electrons
Charge (q): The dance of electrons is measured in Coulombs (C). A single electron carries a charge of 1.60217653×10−19.
Faraday (F): In the grand choreography of biochemical reactions, the Faraday steals the spotlight. It represents the charge of 1 mol elementary charge and is a formidable 9.648534 × 104 C · mol−1 or 96.48596.485 kJ · V−1 · mol−1.
Current (I): The flow of charge is akin to the flow of water. Current, measured in amperes (A), defines the rate of this flow: 1A = 1C⋅s−1.
Voltage and Electrical Work: A Symphony of Energy
Picture the flow of water, a metaphor for electric current. The gravitational potential difference mirrors the electric potential difference between charge "reservoirs." This potential, measured in volts, tells a story of work. A volt (V = 1J⋅C−1) signifies the energy required for a charge of 1 C to move through a potential difference of 1 V. It's a conversion, where energy, work, and charge entwine.
Redox Processes and Electron Carrier Molecules: The Biochemical Ballet
In the biochemical ballet, redox processes take center stage. Electron carriers, the dancers, orchestrate the flow of electrons, paving the way for an electromotive force (emf). Organisms, whether consumers (heterotrophs) or producers (autotrophs), wield molecules as reducing agents, passing electrons from one carrier to another. This dance of electrons follows a rhythm – from lower to higher reduction potentials, generating energy.
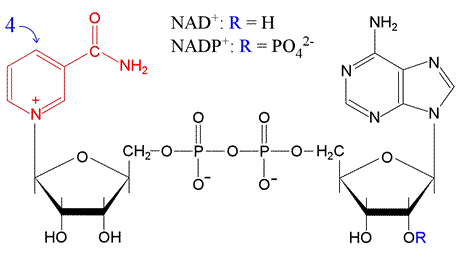
Key Electron Carriers in Biochemistry: NAD and FAD
NAD (Nicotinamide Adenine Dinucleotide):
- Structure: A redox cofactor with NAD+ (oxidized) and NADH (reduced) forms.
- Function: NAD+ accepts electrons in reduction reactions, becoming NADH. It's a key player in catabolic processes.
- Note: NADP+ (with NADPH as the reduced form) is a close relative, often involved in biosynthetic pathways.

- Structure: Comprising an AMP moiety and FMN (flavin mononucleotide), FAD/FMN accepts one or two electrons.
- Function: FAD plays a role in the electron transport chain, accepting electrons and passing them through various complexes.
- Note: FADH2 represents the fully reduced form.
Monitoring Electron Flow: Spectrophotometric Ballet
The progress of redox reactions, especially those involving NAD+/NADH, can be elegantly monitored spectrophotometrically. The appearance of a broad absorption at 340 nm signals the formation of NADH.
Caution: Redox, ROS, and Cellular Harmony
While redox reactions bring harmony to cellular processes, the reduction of molecular oxygen introduces a cautionary note. Reactive oxygen species (ROS), partially reduced oxygen intermediates, can pose a threat. Their reactivity can harm cellular components, emphasizing the delicate balance in the cellular symphony.
In the realm of biochemical redox, where charge flows like a current and electrons dance from carrier to carrier, the elegance of energy transfer unfolds. It's a tale of charge, potential, and carriers, orchestrating the biochemical symphony that sustains life.
|
181 videos|346 docs
|

|
Explore Courses for UPSC exam
|

|