Types and Role in Transport of Oxygen and Carbon Dioxide | Zoology Optional Notes for UPSC PDF Download
Introduction
- Oxygen is essential for ATP generation through oxidative phosphorylation, and therefore must be reliably delivered to all metabolically active cells in the body. In the setting of hypoxia or low blood oxygen levels, irreversible tissue damage can rapidly occur. Hypoxia can result from an impaired oxygen-carrying capacity of the blood (e.g., anemia), impaired unloading of oxygen from hemoglobin in target tissues (e.g., carbon monoxide toxicity), or from a restriction of blood supply. Blood becomes typically saturated with oxygen after passing through the lungs, which have a vast surface area and a thin epithelial layer that allows for the rapid diffusion of gasses between blood and the environment. Oxygenated blood returns to the heart and is distributed throughout the body by way of the systemic vasculature.
- Oxygen is carried in the blood in two forms. The vast majority of oxygen in the blood is bound to hemoglobin within red blood cells, while a small amount of oxygen is physically dissolved in the plasma. The regulation of unloading of oxygen from hemoglobin at target tissues is controlled by several factors, including oxygen concentration gradient, temperature, pH, and concentration of the compound 2,3-Bisphosphoglycerate. The most critical measures of adequate oxygen transportation are hemoglobin concentration and oxygen saturation; the latter is often measured clinically using pulse oximetry.
- Understanding oxygen transport informs our understanding of the underlying mechanisms of tissue hypoxia, ischemia, cyanosis, and necrosis and management to improve global hypoxemia.
Issues of Concern
The transport of oxygen is fundamental to aerobic respiration and the survival of complex organisms. The lungs, heart, vasculature, and red blood cells play essential roles in oxygen transport. Oxygen-carrying deficiencies or problems with oxygen transport or delivery are common sequelae of medical illness and must be promptly evaluated and corrected to prevent irreversible tissue damage.
Organ Systems Involved
- The lungs are the respiratory organs responsible for the exchange of gasses between the bloodstream and the atmosphere. Venous blood entering the lungs typically has a partial pressure (PO) of 40 mm Hg. Upon passing through the alveolar and pulmonary capillaries, oxygen and carbon dioxide are allowed to equilibrate across the blood-air barrier, resulting in carbon dioxide removal from the blood and oxygen absorption. Typically, arterial blood leaving the lungs has a PO of approximately 100 mg Hg. Oxygenated blood is carried through the cardiovascular system to peripheral tissues. In tissues, oxygen diffuses down its concentration gradient from high to low concentrations and is delivered to cells. In the cell, it will act as the terminal electron acceptor in generating adenosine triphosphate (ATP) through oxidative phosphorylation.
- Many organs possess compensatory mechanisms for hypoxia. The compensatory mechanism most relevant to the discussion of oxygen transport is the production of the hormone erythropoietin (EPO) by peritubular fibroblasts in the renal cortex. Erythropoietin stimulates the proliferation and differentiation of red blood cells (erythrocytes) in the red bone marrow--a process known as erythropoiesis. The process of erythropoiesis will increase the number of erythrocytes and subsequent increase in total hemoglobin, which both contribute to an increase in the blood's oxygen-carrying capacity.
Mechanism
Hemoglobin (Hgb or Hb) is the primary carrier of oxygen in humans. Approximately 98% of total oxygen transported in the blood is bound to hemoglobin, while only 2% is dissolved directly in plasma. Hemoglobin is a metalloprotein with four subunits composed of an iron-containing heme group attached to a globin polypeptide chain. One molecule of oxygen can bind to the iron atom of a heme group, giving each hemoglobin the ability to transport four oxygen molecules. One molecule of oxygen can bind to the iron atom of a heme group, giving each hemoglobin the maximum capacity to transport four oxygen molecules. This ability to sequentially bind oxygen to each subunit results in the unique sigmoidal shape of the oxyhemoglobin dissociation curve. Various defects in the synthesis or structure of erythrocytes, hemoglobin, or the globin polypeptide chain can impair the oxygen-carrying capacity of the blood and lead to hypoxia.
The body maintains adequate oxygenation of tissues in the setting of decreased PO or increased demand for oxygen. These changes are often expressed as shifts in the oxygen dissociation curve, representing the percentage of hemoglobin saturated with oxygen at varying levels of PO. Factors that contribute to a right shift in the oxygen dissociation curve and favor the unloading of oxygen correlate with exertion. These include increased body temperature, decreased pH (due to increased production of CO2), and increased 2,3-BPG. (Figure) This right shift of the oxyhemoglobin curve can be viewed as an adaptation for physical exertion. Regulation of the unloading of oxygen from the red blood cells to the target tissues is mainly by the concentration of 2,3-bisphosphoglycerate (2,3-BPG) within erythrocytes. 2,3-BPG preferentially binds to and stabilizes the deoxygenated form of hemoglobin, resulting in a lower affinity of hemoglobin for oxygen at a given oxygen tension and a subsequent increase in the availability of free oxygen for consumption by metabolically active tissues.
Another aspect of oxygen transport is the delivery of oxygen to the tissues each minute. This oxygen delivery depends on both cardiac output (CO) and the arterial oxygen content (CaO):
DO2 = CO * CaO
Note: the CaO calculation is given later in this article. Thus, changes in cardiac output, hemoglobin saturation, and hemoglobin concentration all affect oxygen delivery.
Related Testing
Oxygen is measured in the blood in three ways: partial pressure of dissolved oxygen, oxygen concentration, and hemoglobin saturation. Dissolved oxygen is obtained from arterial blood gas (ABG) measurements and is reported as partial pressure. Henry's law dictates that the amount of dissolved oxygen in plasma water equals the PO times the solubility constant of oxygen in the blood, which is determined to be 0.003 mL / mmHg O / dL blood. This PO is 40 mmHg in the venous and 100 mmHg in the arterial blood. Oxygen first has to dissolve in blood before it can bind to hemoglobin. The amount of dissolved O2 depends on the oxygen gradient between the alveoli and blood and the ease at which oxygen can move through the alveolar lung tissue itself, also known as the parameters involved in Fick's law of diffusion.
The most critical clinical test in assessing the efficacy of oxygen transportation is the concentration of oxygen (CaO). Most oxygen in the blood is bound to hemoglobin, while a minimal amount dissolves in plasma water. Furthermore, the oxygen-carrying capacity of hemoglobin is empirically determined to be 1.34 mL O2 / g Hbg. Thus, when the hemoglobin concentration, hemoglobin saturation (SaO), and PO are known, we can calculate the total oxygen concentration of the blood using the following equation:
CaO = 1.34 * [Hgb] * (SaO / 100) + 0.003 * PaO2.
The saturation of hemoglobin (SaO2) is another measure of the efficacy of oxygen transport and is the ratio of oxygen bound to hemoglobin divided by the total hemoglobin. Saturation can be determined noninvasively in a clinical setting through the use of pulse oximetry, which measures differences in absorption of specific wavelengths o flight by oxygenated and deoxygenated hemoglobin in the blood. Normal levels should be about 80-100% oxygen saturation of Hb. This technique's limitations are that it is a ratio tied to total hemoglobin and thus cannot detect anemia or polycythemia. Additionally, pulse oximetry cannot detect anemia or that oxygenated hemoglobin is indistinguishable from hemoglobin bound to carbon monoxide. Therefore, a person who has suffered exposure to high levels of carbon monoxide may have a normal oxygen saturation as indicated by pulse oximetry, despite lower levels of oxygen bound to hemoglobin.
Pathophysiology
- A persistent reduction in oxygen transportation capacity is most often the result of anemia. The definition of anemia is a decrease in the total amount of hemoglobin in the blood (generally less than 13.5 g / dL in males and 12.5 g / dL in females), which results in reduced carrying capacity for oxygen. Anemia can result from disorders leading to the impaired production of hemoglobin (e.g., iron, B12, or folate deficiency) or the accelerated destruction of hemoglobin, often resulting from a defect in hemoglobin structure.
- Thalassemias are an essential class of inherited disorders resulting in the defective production of hemoglobin. An individual with thalassemia has a mutation that impairs the production of the globin polypeptide chain of hemoglobin. Thalassemias are classified based on the number of genes mutated or absent and whether they encode the alpha or beta-globin chains. While the presentations and severity of thalassemias vary significantly, they all result in a quantitative defect in hemoglobin production.
- Sickle cell anemia ranks as one of the more notable disorders of hemoglobin structure. While the quantity of hemoglobin produced may be normal, a single amino acid substitution of valine for glutamic acid results in a structural defect that promotes the polymerization of deoxygenated hemoglobin. When deoxyhemoglobin polymerizes, it forms fibers that alter the shape of erythrocytes in a process known as sickling. Eventually, repeated stress caused by sickling will damage the membranes of circulating erythrocytes, leading to premature cell death. While sickle cell anemia can remain asymptomatic for a significant time, severe hypoxia may precipitate a sickling crisis, leading to symptoms of generalized pain, fatigue, headache, and jaundice.
- Other defects in oxygen transportation may result from an environmental toxin, with one example being carbon monoxide poisoning, also known as carboxyhemoglobinemia. The affinity of carbon monoxide for hemoglobin is 210 times that of oxygen.The binding of carbon monoxide to hemoglobin leads to a drastic left shift in the oxygen-hemoglobin dissociation curve, impairs oxygen molecules' unloading ability bound to other heme subunits. It is important to note that in the setting of carboxyhemoglobinemia, it is not a reduction in oxygen-carrying capacity that causes pathology but rather an impaired delivery of bound oxygen to target tissues.
Clinical Significance
The primary function of the cardiorespiratory system is to ensure that all metabolically active tissues are adequately oxygenated at all times. Hypoxemia and hypoxia may result when these systems fail and represent major immediate threats to organ function and patient survival. The oxygenation process can be categorized into three stages: oxygenation, oxygen delivery, and oxygen consumption. Respiratory failure will result in a decreased oxygenation of blood. Oxygen delivery, the rate of oxygen transport from the lungs to the microcirculation, is dependent on cardiac output and arterial oxygen content. And oxygen demand is a product of the metabolic state of the tissues. All three of these processes must be evaluated and corrected in the clinical setting, particularly in critically ill patients or acute situations. The management of hypoxia typically involves efforts to improve global hypoxemia, oxygen delivery and focuses on blood oxygenation through supplemental oxygen and positive-pressure ventilation and cardiac output.Transport of Carbon Dioxide in the Blood
Carbon dioxide molecules are transported in the blood from body tissues to the lungs by one of three methods: dissolution directly into the blood, binding to hemoglobin, or carried as a bicarbonate ion. Several properties of carbon dioxide in the blood affect its transport. First, carbon dioxide is more soluble in blood than oxygen. About 5 to 7 percent of all carbon dioxide is dissolved in the plasma. Second, carbon dioxide can bind to plasma proteins or can enter red blood cells and bind to hemoglobin. This form transports about 10 percent of the carbon dioxide. When carbon dioxide binds to hemoglobin, a molecule called carbaminohemoglobin is formed. Binding of carbon dioxide to hemoglobin is reversible. Therefore, when it reaches the lungs, the carbon dioxide can freely dissociate from the hemoglobin and be expelled from the body.
Third, the majority of carbon dioxide molecules (85 percent) are carried as part of the bicarbonate buffer system. In this system, carbon dioxide diffuses into the red blood cells. Carbonic anhydrase (CA) within the red blood cells quickly converts the carbon dioxide into carbonic acid (H2CO3)(H2CO3). Carbonic acid is an unstable intermediate molecule that immediately dissociates into bicarbonate ions (HCO−3)(HCO3−) and hydrogen (H+) ions. Since carbon dioxide is quickly converted into bicarbonate ions, this reaction allows for the continued uptake of carbon dioxide into the blood down its concentration gradient. It also results in the production of H+ions. If too much H+ is produced, it can alter blood pH. However, hemoglobin binds to the free H+ ions and thus limits shifts in pH. The newly synthesized bicarbonate ion is transported out of the red blood cell into the liquid component of the blood in exchange for a chloride ion (Cl−); this is called the chloride shift. When the blood reaches the lungs, the bicarbonate ion is transported back into the red blood cell in exchange for the chloride ion. The H+ ion dissociates from the hemoglobin and binds to the bicarbonate ion. This produces the carbonic acid intermediate, which is converted back into carbon dioxide through the enzymatic action of CA. The carbon dioxide produced is expelled through the lungs during exhalation.
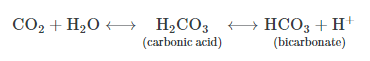
The benefit of the bicarbonate buffer system is that carbon dioxide is “soaked up” into the blood with little change to the pH of the system. This is important because it takes only a small change in the overall pH of the body for severe injury or death to result. The presence of this bicarbonate buffer system also allows for people to travel and live at high altitudes: When the partial pressure of oxygen and carbon dioxide change at high altitudes, the bicarbonate buffer system adjusts to regulate carbon dioxide while maintaining the correct pH in the body.
Carbon Monoxide Poisoning
While carbon dioxide can readily associate and dissociate from hemoglobin, other molecules such as carbon monoxide (CO) cannot. Carbon monoxide has a greater affinity for hemoglobin than oxygen. Therefore, when carbon monoxide is present, it binds to hemoglobin preferentially over oxygen. As a result, oxygen cannot bind to hemoglobin, so very little oxygen is transported through the body (Figure 1).
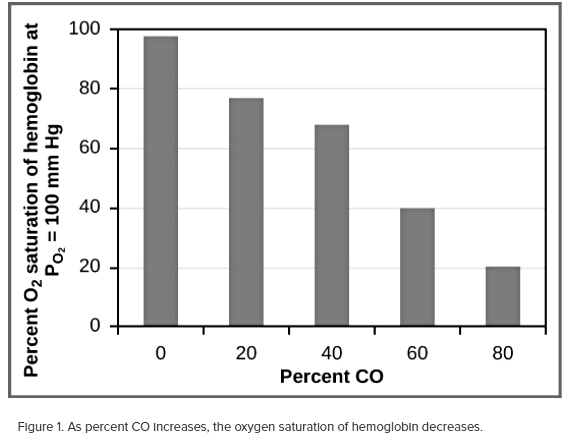
Carbon monoxide is a colorless, odorless gas and is therefore difficult to detect. It is produced by gas-powered vehicles and tools. Carbon monoxide can cause headaches, confusion, and nausea; long-term exposure can cause brain damage or death. Administering 100 percent (pure) oxygen is the usual treatment for carbon monoxide poisoning. Administration of pure oxygen speeds up the separation of carbon monoxide from hemoglobin.
|
181 videos|351 docs
|
















