Protein Folding | Zoology Optional Notes for UPSC PDF Download
Protein Folding and Restructuring
Flexibility of Protein Chains & Structural Ensembles
In structural biology, the prevalent belief is that a protein's sequence dictates its 3D structure, subsequently determining its function. This perspective assumes a rigid view of protein structures, essential for methods such as structure prediction and comparison. However, proteins should be recognized as flexible entities capable of adopting diverse structural conformations. This flexibility depends on various physical conditions and the presence of binding partners.
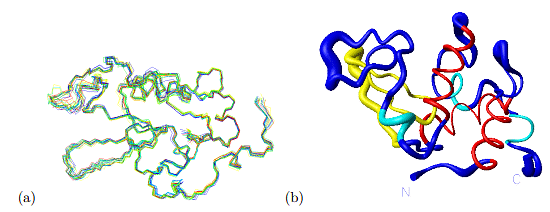
Conformational Ensemble
- Dynamic Nature: Proteins exhibit flexibility, allowing them to exist in an ensemble of structural conformations.
- Environmental Influence: Conformational changes depend on factors like temperature, pH, salt concentration, protein concentration, binding partners, membrane presence, and cell cytoplasm crowding.
- Functional Role: The dynamic interaction between a protein's structural ensemble and its environment is crucial for its functionality.
Importance of Flexibility
- Protein Folding and Allostery: The ability of proteins to change shape is fundamental to processes like protein folding, allostery (conformational change upon ligand-binding), and complex formation.
- Limitation of Methods: Many experimental and bioinformatics methods do not explicitly handle protein flexibility.
Simulation Methods
- Introduction: This section explores simulation methods and elucidates the thermodynamic principles behind them.
- Explicit Modeling: Simulations provide a means to explicitly model protein flexibility, allowing consideration of an ensemble of structural conformations.
- Insight Gained: Simulations, coupled with experimental observations, offer insights into the folding process and how structural flexibility enables protein functionality.
- Dynamic Processes: Protein structure and folding are dynamic and stochastic processes, not deterministic or static.
Defining the Folded and Unfolded States
Folded State Definition
- Intuition: Before delving further, defining the folded (native) state is essential.
- Experimental Determination: Experimentally, folded states resemble a uniquely folded conformation, covering a small ensemble at physiological temperatures.
- Unfolded State Complexity: The unfolded state encompasses a large ensemble of possibly extended conformations, influenced by specific system conditions.
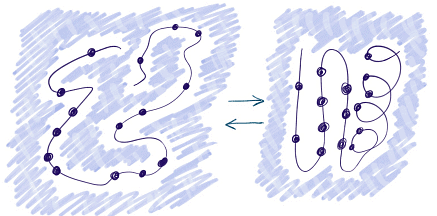
Experimental Observations
- NMR and CD Methods: Techniques like NMR and CD can determine if a solution contains folded proteins.
- Green Fluorescent Protein (GFP): GFP fluorescence indicates a fully folded state, making it useful for folding experiments.
- Enzymatic Activity: Enzyme catalytic activity serves as an indicator of a fully folded native form.
Simulation Assessment
- Comparison Measures: Simulations assess folding by comparing snapshots to experimentally determined structures.
- RMSD and Topological Similarity: Common measures include RMSD to the native structure and topological similarity of internal contacts.
- Ensemble Analysis: The ensemble of conformations dissimilar to the native structure defines the unfolded state.
Folding and Refolding
Dynamic Equilibrium of Protein Folding
Illustrative Scenario
- Dynamic and Flexible Nature: Proteins exhibit dynamic and flexible characteristics.
- In Vitro System: Consider a simple system of proteins in water, two-state folders, rapidly transitioning between folded and unfolded states.
- Dynamic Equilibrium: The system maintains a dynamic equilibrium, with fractions of unfolded and folded proteins stabilizing over time.
- Conformational Ensemble: During unfolding to folding, a molecule explores various conformations but spends the majority of its time in either the folded or unfolded state.
Stability and Probability
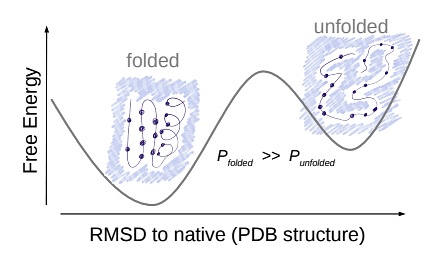
State Probability and Stability Relation
- Observation Significance: The probability of being in a state is linked to the stability of that state.
- Physiological Conditions: At around 30 ◦C, proteins are most stable in their native, folded state.
- Free Energy Difference: The free energy of the folded state is lower than that of the unfolded state.
- Quantitative Relation: The probability of a state is quantitatively related to its free energy.
Changing Conditions
Temperature Impact: Raising the temperature can shift stability, making the unfolded state more favorable.
Entropic Effect: Higher temperatures favor states with many possible conformations, such as the unfolded state.
Other Condition Changes: Altering pH, salt concentration, or adding denaturants can influence the relative stabilities of folded and unfolded states.
Equilibrium vs. Unstable Situation: In equilibrium, fractions of folded and unfolded conformations are determined by state probabilities. In non-equilibrium situations, such as a temperature jump experiment, the system relaxes over time until a new equilibrium is reached.
Factors that (De)Stabilize the Native Fold
Hydrophobic Effect
- Stabilizing Effect: Hydrophobic side chain burial is crucial for stabilizing globular proteins.
- Water Attraction: Water molecules favor forming hydrogen bonds, and hydrophobic particles cannot participate.
- Interface Formation: Unfolded states lead to hydrophobic residues interfacing with water, while folded states avoid this, making the folded state more favorable.
Hydrogen Bonds, Salt-Bridges, and Packing
- Additional Stabilizing Factors: Van der Waals forces, hydrogen bonds, salt bridges, and quantum effects contribute to native state stability.
- Detailed Interactions: Interactions between side chain and backbone atoms play a role.
- Quantum Effects: π-π interactions involving aromatic residues contribute to stability.
Backbone Entropy
- Entropy Favoring Unfolded State: The entropy of the backbone favors unfolded states due to numerous possibilities.
- Conformational Flexibility: Unfolded conformations have more possibilities than exact matches to native conformations.
- Overall Stability Balance: If other factors don't favor the folded state, proteins remain unfolded.
Anfinsen’s Theorem
- Determinants of Protein Structure: Solvent properties, temperature, pH contribute to structure, but amino acid sequence is the primary determinant.
- Denaturation-Renaturation Experiments: Anfinsen's experiments showed proteins can be denatured and spontaneously refold to native forms with changing conditions.
- Thermodynamic Hypothesis: Folded structure encoded by sequence, found due to thermodynamic laws.
- Conditions for Thermodynamic Hypothesis: Uniqueness, stability, and kinetic accessibility of the free energy minimum are crucial.
- Functional State Variations: While true for most proteins, some functional states observed in nature may not have the lowest free energy in in vitro experiments.
Folding Pathways
Complex Folding Pathways
- Two-State Folders: Previously considered a simple scenario with two stable states: folded and unfolded.
- Complex Pathways: Many proteins have more complex folding pathways with intermediate stable states.
- Multi-Domain Proteins: Examples include multi-domain proteins folding one domain at a time in a specific order.
- Existence of Foldons: Smaller intermediate folding structures or foldons as meta-stable states on the folding path.
Experimental Observations
- Diverse Pathways: Different proteins exhibit diverse folding pathways experimentally.
- Stochastic Nature: Generally, folding and refolding are stochastic processes.
- Further Exploration: Detailed exploration of stochastic processes will be covered in the last section of this chapter.
Free Energy Landscapes
- Simple Free Energy Landscape: Described using Figure 12.3, depicting two-state processes like protein folding.
- Transition State: Two free energy minima separated by a barrier, with the top of the barrier as the transition state.
- Barrier Height Importance: Barrier height determines the rate of transition between states.
- Challenge in Simulations: Sampling the transition state is challenging due to its instability, often leading to a bias towards the lower energy minimum.
Levinthal’s Paradox
- Spontaneous Folding Dilemma: Levinthal proposed a paradox that spontaneous folding would take more time than the age of the universe.
- Protein Conformational Possibilities: Considered a protein with 100 amino acids, each with two torsion angles and three possible values.
- Impractical Sampling: If each conformation takes one picosecond to visit, it would take 10^75 years to explore all possibilities.
- Resolution: Levinthal's paradox suggests that folding proteins sample only a small fraction of possible conformations before reaching the most stable state.
- Smooth Folding Pathways: A typical protein's folding path is relatively smooth, allowing for early stable interactions.
Folding in the Cell
In Vivo Folding
- Cellular Context: Inside the cell (in vivo), folding occurs during and after the synthesis of polypeptide chains in the ribosomes.
- Biological Function Necessity: Correct folding is crucial for proteins to perform their biological function.
- Reversibility Assumption: Protein folding assumed to be reversible, but some proteins may not refold after unfolding.
- Variability: Proteins may fold directly during synthesis, require chaperones, or fold upon binding specific partners.
Chaperones
- Chaperone Assistance: Chaperones aid in preventing misfolding and aiding proper folding.
- Heat-Shock Proteins: Most chaperones, like GroEL, are heat-shock proteins upregulated as temperature increases.
- Multiple Functions: Besides folding assistance, chaperones may prevent aggregation of misfolded proteins.
Marginally Stable Folded Proteins
- Marginal Stability: Many naturally occurring proteins are only marginally stable under physiological conditions.
- Evolutionary Balance: Evolutionary balance between stability and flexibility, sufficient for function but allowing movement.
- Degradation Consequence: Increased stability may result in additional costs during degradation in the ubiquitin-mediated proteasome.
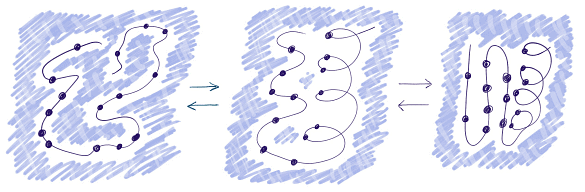
Alternative Stable States of Proteins
Molten Globules
- Compact Unfolded State: Molten globule state is compact, with clustered hydrophobic amino acid residues.
- Hydrophobic Collapse Concept: Molten globules may be more stable than the folded state under specific conditions or meta-stable states along a folding pathway.
Natively Disordered Proteins
- Functional Unfolded Proteins: Natively, intrinsically disordered proteins maintain (largely) unfolded functional conformations.
- Previously Discussed: Covered in Chapter “Protein Function & Interactions” Section 3.5.
Misfolding
- In Vivo Misfolding: Cellular processes may occasionally fail, leading to protein misfolding.
- Functional Implications: Misfolded states may not be functional, kinetically trapped, and potentially dangerous within the cell.
- Possible Causes: Chance misfolding, abrupt environmental changes, or interactions with other proteins (e.g., prions).
Aggregation and Amyloid Formation
- More Dangerous Misfolding: Aggregation of multiple protein molecules, leading to amyloid fibrils.
- Stability Comparison: Amyloid state may be more stable than the folded state under certain conditions.
- Long-Term Consequence: Over time, proteins may aggregate into amyloid fibrils, potentially causing cellular disruptions.
- Prion Example: Misfolding of prion proteins can lead to disruptive β fibrils.
Protein Folding in Experiment and Simulation
Experimental Challenges
- Observing Intermediate Stages: Experimentally challenging to observe intermediate stages of the folding pathway.
- Structural Information: Techniques like X-ray crystallography, NMR, and spectroscopy offer structural information but limited insight into dynamic processes.
Computational Simulations
- Understanding Mechanisms: Computational simulations, like molecular dynamics (MD), provide insights into the underlying mechanisms of protein folding.
- Limitations: Full folding simulation of large proteins is challenging due to computational time and model accuracy constraints.
- Simulation Achievements: Successful simulations for small peptides and proteins, providing valuable insights when combined with experimental observations.
Key Concepts
Protein Conformations
- Diverse Conformations: Proteins have the ability to adopt numerous conformations.
- Structural Variety: This diversity in conformation contributes to the structural complexity and functional versatility of proteins.
Native State Stability
- Physiological Conditions: Under physiological conditions, the native (functional) state of proteins is usually the most stable.
- Functional Importance: Stability of the native state is crucial for the protein to carry out its biological function effectively.
Dynamic Equilibrium
- Continuous Dynamics: Proteins exhibit continuous dynamics, undergoing processes of folding, unfolding, and re-folding.
- Dynamic Equilibrium: This continuous dynamic behavior establishes a dynamic equilibrium in which the fractions of folded and unfolded states remain stable over time.
Stochastic Nature of Protein Folding
- Stochastic Process: Protein folding is a stochastic process, meaning it involves random elements.
- Non-Deterministic: The process lacks determinism, and the outcome is influenced by probabilistic events rather than predetermined steps.
Cellular Environment Challenges
- Crowded Cellular Environment: Within the crowded environment of the cell, proteins face challenges in folding properly.
- Misfolded Protein Accumulation: Special precautions are necessary to prevent problems arising from the accumulation of misfolded proteins, which can be disruptive in the cellular milieu.
Temperature Influence on Stability
- Temperature and Entropy: Increasing temperature enhances the stability of the entropically favorable state, typically the unfolded state in protein folding.
- Entropic Favorability: Higher temperatures favor states with greater entropy, and in protein folding, this aligns with the unfolded state.
- Temperature and Enthalpy: Conversely, decreasing the temperature increases the stability of the enthalpically favorable state.
- Enthalpic Favorability: Lower temperatures promote states with lower enthalpy, and in protein folding, this is often associated with the folded state.
|
181 videos|351 docs
|















