Grade 7 Exam > Grade 7 Notes > Science for Grade 7 > Olympiad Notes: Matter and Materials
Olympiad Notes: Matter and Materials | Science for Grade 7 PDF Download
Introduction to Matter

- Definition: Matter is anything with space occupancy, mass, and sensory perception.
- Indestructibility: Matter can neither be created nor destroyed.
Understanding Materials
- Definition: Materials are substances like metal, wood, plastic, glass, and fabric, used in making or constructing things.
- Relation to Matter: Materials are forms of matter, which is a physical substance occupying space and possessing weight.
Question for Olympiad Notes: Matter and MaterialsTry yourself: Which of the following best defines matter?View Solution
Physical Properties of Matter
Characteristics: The properties of matter include distinct qualities and features that set different samples apart.
Observation Techniques:
- Look: Noticing size, shape, location, and features.
- Feel: Sensing textures, varying among materials like iron, paper, plastic.
- Smell: Identifying unique scents, such as differences between hair oil, cooking oil, and kerosene.
- Taste: Recognizing distinct tastes, like sugar's sweetness or salt's saltiness. Note: Some substances can be poisonous.
Elements of Matter
- Molecular Structure: Matter is made up of molecular particles.
- Molecules: These are atoms bonded together, invisible to the naked eye.
- Uniqueness: Every substance has uniquely different molecules.
Three States of Matter
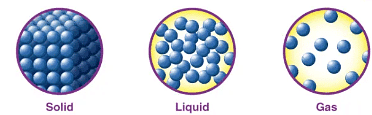
- Solid: Keeps shape and volume, like tin boxes, buildings, stones.
- Liquid: Takes the shape of containers, maintains volume, but can't hold shape independently.
- Gas: Consists of loosely packed particles, fills any available space, and can become liquid under compression.
- Overview: Matter exists as solids, liquids, and gases.
- Common Properties: All states occupy space, have mass, and are perceptible, qualifying as matter.
Question for Olympiad Notes: Matter and MaterialsTry yourself: Which observation technique is used to identify unique scents?View Solution
Characteristics of Each State
- Properties: Fixed mass, volume, shape; rigid.
- Compressibility: Non-compressible.
- Particle Behavior: Limited diffusion and motion; strong particle attraction.
- Examples: Paper, Wood.
Liquids
- Properties: Fixed mass, volume; shapeless.
- Compressibility: Slightly compressible.
- Particle Behavior: Possible diffusion; more motion than solids.
- Examples: Water, Alcohol.
Gases
- Properties: Fixed mass; shapeless, volumeless.
- Compressibility: Highly compressible.
- Particle Behavior: Rapid diffusion; minimal attraction; maximum motion.
- Examples: Hydrogen, Carbon Dioxide.
Demonstrations of State Properties
- Experiment: Metal rods in various containers with water.
- Conclusion: Solids retain shape and volume; liquids adopt container shape but keep volume.
Volume and Shape of Gases
- Experiment: Inverted gas jar with colored gas over a beaker.
- Conclusion: Gases fill and shape their container.
Compressibility of Solids and Gases
- Experiment: Pressing wood and squeezing bottles with water and gas.
- Conclusion: Solids are non-compressible; gases are highly compressible.
Attraction Between Solid Molecules
- Experiment: Merging mercury globules.
- Conclusion: Solid particles show attraction.
Motion of Molecules
- Experiment: Potassium permanganate in water, perfume dispersion, talcum powder in water.
- Conclusion: Constant molecular motion.
Interconversion of Matter
- Concept: Matter changes states influenced by temperature and pressure.
The document Olympiad Notes: Matter and Materials | Science for Grade 7 is a part of the Grade 7 Course Science for Grade 7.
All you need of Grade 7 at this link: Grade 7
|
75 videos|160 docs|52 tests
|
FAQs on Olympiad Notes: Matter and Materials - Science for Grade 7
| 1. What is matter? |  |
Ans. Matter is anything that has mass and occupies space. It can exist in three states - solid, liquid, and gas. Matter is made up of tiny particles called atoms and molecules.
| 2. What are physical properties of matter? |  |
Ans. Physical properties of matter are characteristics that can be observed or measured without changing the substance's composition. Examples of physical properties include color, density, melting point, boiling point, and conductivity.
| 3. What are the three states of matter? |  |
Ans. The three states of matter are solid, liquid, and gas. In the solid state, particles are tightly packed and have a fixed shape and volume. In the liquid state, particles are loosely packed and have a definite volume but no fixed shape. In the gas state, particles are widely spaced and have neither a fixed shape nor volume.
| 4. How do the characteristics of each state of matter differ? |  |
Ans. The characteristics of each state of matter differ in terms of particle arrangement, energy, and movement. In the solid state, particles are closely packed and vibrate in fixed positions. In the liquid state, particles are more loosely packed and can move past each other. In the gas state, particles are far apart and move freely in all directions.
| 5. Can matter change from one state to another? |  |
Ans. Yes, matter can change from one state to another through physical processes such as heating or cooling. This is known as the interconversion of matter. For example, when a solid is heated, it can melt into a liquid, and when a liquid is cooled, it can freeze into a solid. Similarly, a liquid can evaporate into a gas when heated, and a gas can condense into a liquid when cooled.
Related Searches





















