Antiaromatic Compounds and Antiaromaticity | Chemistry Optional Notes for UPSC PDF Download
Spectacularly Unstable Molecules That Form A Category Unto Themselves: “Anti-Aromatic”
Here’s the thing. There’s a small number of molecules that flunk the aromaticity test that aren’t just non-aromatic: they have the property of being so spectacularly and unusually unstable and difficult to isolate that they deserve another name.
We call these molecules “anti-aromatic“.
What’s interesting is that in many cases the molecules themselves don’t look to be particularly unstable from first principles, in contrast with, say, cubane. Funny enough, you can buy cubane (more or less) from Aldrich, but most of the anti-aromatic molecules we’ll discuss below are only stable at extremely low temperatures – if they can be isolated at all!
Cyclopentadienyl Cation: The “Resonance-Stabilized” Carbocation That Isn’t Stable
Let’s start our journey down the rabbit hole with a simple example that should seem familiar from first-semester organic chemistry.
Quick review: Start with an alkyl halide. Leaving group leaves, forming a carbocation. Nucleophile attacks. It’s called the SN1 because the rate-determining step (carbocation formation) is unimolecular. Bottom line: the more stable the carbocation, the faster the reaction proceeds. Hence, the reaction rate for alkyl halides is tertiary > secondary > primary, and the carbocation gets special bonus points if it’s stabilized by resonance.
Look at these two SN1 reactions. Which one do you think would happen faster – the SN1 reaction which goes through a secondary carbocation (A), or the SN1 reaction which goes through a secondary carbocation and can form multiple resonance forms (B)?
From everything you’ve learned so far, you’d expect the answer to be B).

It’s not.
The answer is that reaction A) happens faster. Reaction B does not happen at all.
This should strike you as deeply weird.
It is!
How is it that this supposedly resonance-stabilized carbocation is less stable than a secondary carbocation?
After all, how many times is it drilled into your head that resonance stabilizes molecules, especially carbocations?
But here, it seems to be destabilizing. It’s… it’s…
But unstable it is! The cyclopentadienyl cation is incredibly unstable and difficult to make – and it’s not for lack of trying. There’s something very strange about the structure of the cyclopentadienyl cation that gives it unusual instability.
The cyclopentadienyl cation is unusually unstable

Cyclic, conjugated, flat…. and has 4 pi electrons. Interesting!
Antiaromatic Three-Membered Rings: Oxirene, 1H-Azirene, Thiirene
Another class of “elusive” molecules is a family of three-membered rings. You might recall from Org 1 that it’s easy to make epoxides from alkenes using an oxidant like m-CPBA.
Did you ever wonder why we never covered the same reaction for alkynes?
Well, it’s not for lack of trying. Chemists have tried all kinds of methods for epoxidizing alkynes, and you know what? The reaction just doesn’t frickin’ work.
For instance, epoxidation of acetylene would give the molecule below (oxirene).
Oxirene itself has never been observed, although there are tantalizing traces of its fleeting existence. And by the way, neither has the nitrogen analogue, 1H-azirene, or the thiirene.
Some Elusive Three-Membered Rings

Why not? What’s special about each of these cases?
You might notice that, like the cyclopentadienyl cation, these molecules are cyclic, conjugated, flat, and have 4 pi electrons (two in the pi bond, and two from a lone pair).
OK. What else might be cyclic, conjugated, flat, and have 4 pi electrons?
Cyclobutadiene Is Antiaromatic
Cyclobutadiene looks like a simple enough molecule, but it wasn’t actually synthesized until 1965. And even then, it was found that it isn’t stable at temperatures above 35 Kelvin.
The question is, why?
Sure, it’s a four-membered ring, and yes, it has a lot of ring strain, but more strained molecules have been made that are actually stable at room temperature.
You might also note that like the above examples, cyclobutadiene is another example of a molecule that is cyclic, conjugated, has 4 pi-electrons, and is flat.
What’s even more interesting is what was learned about the geometry of cyclobutadiene. Rather than being a molecule with identical bond lengths (like benzene), cyclobutadiene was found to have a rectangular shape, indicating that the electrons were not delocalized.

What this tells us is that even when a molecule fulfills all the conditions (cyclic, conjugated, flat, 4 pi electrons), the symmetric geometry is particularly unstable.
An 8 Pi-Electron Example of an Antiaromatic Compound: Pentalene
So far all the examples we’ve seen so far had 4 pi electrons. You might be wondering: are there any examples of anti-aromatic molecules that have more than 4 pi electrons? Why, yes.
The molecule below is called “Pentalene”. It has been synthesized, but is only stable below –100 °C. Above this temperature it combines with another molecule of itself. Another example of spectacular instability.

Pentalene has 8 pi electrons. This might set off some alarm bells of recognition. Remember how the number of pi-electrons in aromatic molecules followed the sequence (2, 6, 10, 14….) ?
This example suggests that the number of pi electrons in anti-aromatic molecules follows the sequence (4, 8, 12…)
What Makes A Molecule Anti-Aromatic?
So what do all of these molecules in this rogues’ gallery have in common?
Each of them is cyclic, conjugated, and flat – and when you count the number of pi electrons, it’s multiples of 4. So while aromatic molecules have (4n+2) pi electrons, the “rule” for anti aromatic molecules is (4n). (another way to look at it: the number of pi electrons will be twice an even number).
- Each of these unusually unstable molecules is cyclic, conjugated, and flat.
- The number of Pi electrons is 4 or 8 & (=4n)

we call this unusual instability anti-aromaticity
This means that we can now draw up three categories for molecules according to the following criteria:

- Aromatic molecules are cyclic, conjugated, have (4n+2) pi electrons, and are flat.
- Anti-aromatic molecules are cyclic, conjugated, have (4n) pi electrons, and are flat.
- Non-aromatic molecules are every other molecule that fails one of these conditions.
Wait a second – you might wonder why cyclooctatetraene is classified as “non-aromatic”. With 8 pi electrons (twice an even number) shouldn’t it be “anti-aromatic”?
Cyclooctatetraene “Escapes” Anti-Aromaticity Through Twisting Out of Flatness
Think of fulfilling the conditions for “anti-aromaticity” as a bit like qualifying for an extremely punishing income tax. Given the opportunity to find a loophole to get out of the tax, would you do it? Probably.
Cyclooctatetraene is anti-aromatic only if it is flat. However, the relatively “floppy” structure of cyclooctatetraene allows for some flexibility. The bonds can rotate away from flatness such that the molecule adopts a “tub-like” shape, thereby avoiding the “antiaromaticity tax” of 12 kcal/mol that would be paid if all the p-orbitals on the molecule were conjugated with each other.
WAIT! Isn't cyclooctatetraene anti-aromatic?
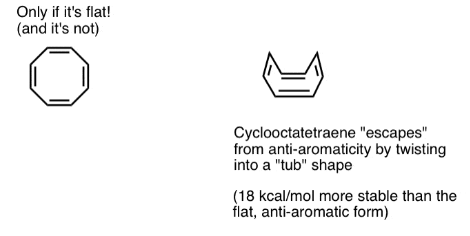
It turns out that cyclooctatetraene has been synthesized, is stable (you can buy it here, for example) and behaves like a “normal” alkene, undergoing addition reactions, hydrogenation, and so on.
Pentalene (above) which also has 8 pi-electrons, has a very rigid bicyclic structure that prevents bond-rotation away from flatness. Hence, it’s stuck in its anti-aromatic conformation.
Anti-aromaticity for molecules with more than 8 pi electrons is known, but very unusual.
Conclusion: Antiaromatic Compounds
So far our treatment of aromaticity and anti-aromaticity has been purely descriptive and empirical. We’ve shown lots of examples, and given lots of rules, but missing from the discussion has been any deep explanation of “why“.
What’s so special about the benzene ring that makes it so stable? Why is it stable?
What’s so special about the cyclobutadiene system that makes it so unstable. Why is it unstable?
In order to answer these deeper questions, we’re going to have to step back and examine the molecular orbitals of these two molecules, and then come to a deeper understanding of aromaticity and anti-aromaticity.
FAQs on Antiaromatic Compounds and Antiaromaticity - Chemistry Optional Notes for UPSC
| 1. What are antiaromatic compounds? |  |
| 2. Why are cyclopentadienyl cation and cyclobutadiene considered antiaromatic? |  |
| 3. How does cyclooctatetraene escape antiaromaticity? |  |
| 4. What are some examples of antiaromatic three-membered rings? |  |
| 5. Why are antiaromatic compounds highly unstable? |  |

|
Explore Courses for UPSC exam
|

|
















