Azulenes: Synthesis and Reactions | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Azulene |

|
| Occurrence |

|
| Synthesis |

|
| Azulene Syntheses From Cycloheptanone |

|
| Reactions of Azulenes |

|
| Annulenes |

|
| General Synthesis of Annulenes |

|
Azulene
- Azulene - non-benzenoid aromatic compound which has a fused structure of five- and seven-membered rings.


- It is a structural isomer of naphthalene, however, they differ in physical and chemical properties:
- Naphthalene- colourless while, azulene - deep blue colour
- Naphthalene has zero dipole moment while, azulene has a dipole moment of 1.08D
- Naphthalene is more stable than azulene
• The ionic cyclopentadienide and tropylium structures contribute to the azulene skeleton (as shown in fig. below).
• 1- and 3-positions are sites of electrophilic substitution reactions and 4-, 6-, and 8-positions are sites of nucleophilic addition.
• Azulene is blue in colour and has large dipole moment of 1.08 D (unusual for small aromatic compounds)- this is due to the intramolecular charge transfer from the dipolar resonance structure as shown below.

Occurrence
- A variety of azulenes have been found in essential oils (anise and fennel seed oil, peppermint oil, vetiver oil (kush oil) etc.) and other natural products.
- The anti-inflammatory action of camomile oil is due to the presence of azulene in it. Camomile extracts have no such physiological action until they are distilled to form the azulene, this confirms the role of azulene in anti-inflammatory action
Synthesis
- The first synthesis of azulene was carried out in 1893 by dry distillation of calcium adipate, however, yield was poor.

- The first total azulene synthesis was carried out by Pfau and Plattner (1936) from cyclopentenocycloheptanone, made from 1,6-cyclodecadione.

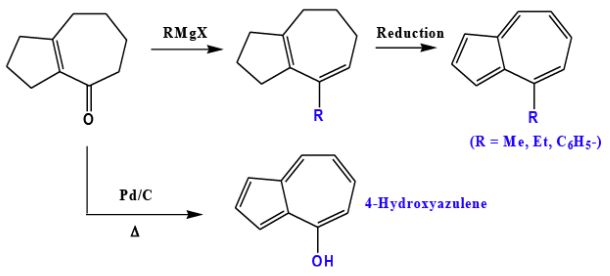
Azulene Syntheses From Cycloheptanone
- Cyclopentane ring is built up on cycloheptanone and then the product is dehydrogenated.
- Well suited for 1- and 2-substituted azulenes.
In the example given below, preparation of 1-alkylazulenes is carried out by modified Stobbe condensation.

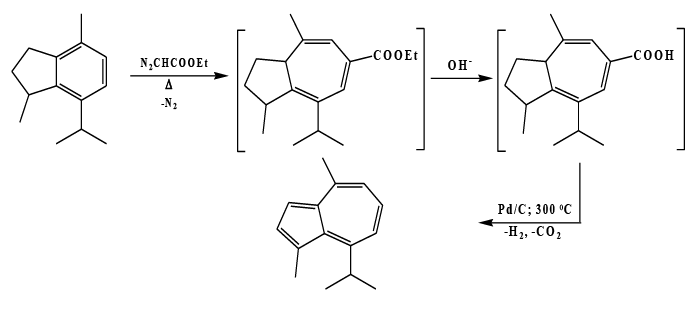
Synthesis of 4-Substituted azulenes
By ring expansion reaction of indane derivatives
Reactions of Azulenes
- Electrohilic substitution occurs on the 5-membered ring
- Friedel-Crafts acetylation of azulene gives 1-acetylazulene and a 1,3-diacetylazulene
- Nitration- Treatment of azulene with cupric nitrate and acetic anhydride gives 1-Nitroazulene

- Azulene ketone can form oxime which undergoes Beckmann rearrangement

- Nucleohilic substitution occurs on the 7-membered ring

Annulenes
- Annulenes are completely conjugated monocyclic hydrocarbons.
- General formula CnHn ( n is an even number) or CnHn+1 (when n is an odd number)
- Out of the first eight even annulenes, only [6]annulene (Benzene) and [18]annulene are aromatic.
- Thus, of the first eight even annulenes only [14]annulene and [18]annulene are non-benzenoid aromatics.

- Due to aromatic character, 1H NMR of [14]- and [18]annulene shows two sets of signals due to ring current. The protons on the periphery (outer) show downfield shift while the interior (inner) protons show upfield shift.
- These differences are observed only at low temperatures. A single resonance peak is observed in 1H NMR spectrum of [18]annulene at room temperature, This is due to rapid equilibration of the inside and outside Hs due to cis-trans interconversion about the double bonds.

General Synthesis of Annulenes
Involves three steps:
- Oxidative coupling of a suitable terminal diacetylene gives macrocyclic polyacetylene of required ring size, generally copper(II) acetate in pyridine is used for this transformation.
- Transformation of macrocyclic polyacetylene to a dehydroannulene, usually by prototropic rearrangement effected by potassium tert-butoxide.
- In the final step, partial catalytic hydrogenation of the triple bonds to double bonds gives the desired annulene. [14]-, [16]-, [18]-, [20]-, [22]-, and [24]annulenes have been prepared in pure crystalline form by this method


FAQs on Azulenes: Synthesis and Reactions - Chemistry Optional Notes for UPSC
| 1. What is the occurrence of azulene? |  |
| 2. How is azulene synthesized from cycloheptanone? |  |
| 3. What are the reactions of azulenes? |  |
| 4. What are annulenes? |  |
| 5. How are annulenes synthesized? |  |

|
Explore Courses for UPSC exam
|

|