Chelating Ligands and the Chelate Effect | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Chelating Ligands |

|
| Introduction |

|
| Denticity |

|
| Coordination isomers in polydentate ligands |

|
| Bite Angle |

|
| The Chelate Effect (and Macrocycle Effect) |

|
| The Chelate Effect |

|
| The Macrocycle Effect |

|
Chelating Ligands
Introduction
- Each ligand that binds to a transition metal donates a pair of its electrons to the metal, sharing those electrons in a metal-ligand bond. Counting the total number of electrons in the metal's valence shell is fairly easy when you know that each ligand contributes two electrons.
- But what if a ligand could contribute more than two electrons? Take a look at the following table. Each of the ligands shown here can bind to a metal twice. The ligand forms two bonds to the metal, donating two pairs of electrons. It might seem obvious, but forming two bonds to the metal means the ligand binds more tightly to the metal. Remember the lego-like nature of transition metal complexes: ligands can come and go, but these ligands are less likely to go; they hold on.

- These ligands are called bidentate ligands. That means, literally, that they have two teeth. That does not sound like much, but the ligands we have seen previously are described as monodentate; they have only one tooth. Bidentate ligands can bite into and hold onto the metal more strongly than monodentate ligands.
- Another term used for these kinds of ligands is derived from the Greek chele, for claw. These ligands grab onto the metal like the claw of a lobster or crab; they chelate. The "chelate effect" (see next section) is the tendency of these ligands to bind firmly to a metal, whereas monodentate ligands might come off more easily.
Denticity
Denticity refers to the number of atoms with which a ligand binds to a metal ion. A ligand could be monodentate, meaning it binds through a lone pair on a single atom. It could be bidentate, meaning it binds through lone pairs on two different atoms. It could even be tridentate, with three atoms bearing their own lone pairs, tetradentate, and so on. The words, polydentate and multidentate, are two general terms for ligands that bind through more than one atom. The words chelator and chelating ligand are also general terms used to refer to these ligands.
Figures 3.1.1.2 and 3.1.1.3: Examples of polydentate ligands.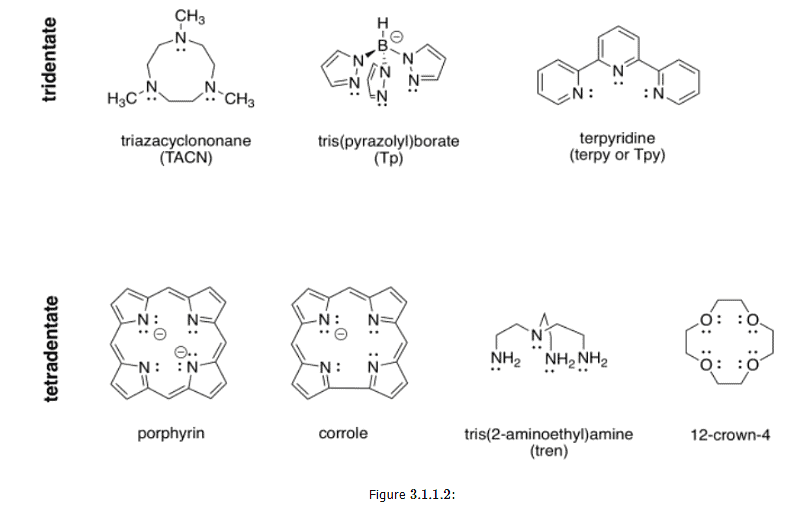

There is a symbol for denticity, κ (it's a Greek letter, pronounced "kappa"), which simply describes how many atoms are bound to the metal. For example, in ethylenediamine or 1,2-diaminoethane, NH2CH2CH2NH2, the two nitrogen atoms can be bound to the metal at the same time, although none of the other atoms in between would be directly attached to the metal. This donor is capable of binding in a κ2 mode. However, if for some reason one of the nitrogen atoms lets go of the metal so that the ethylenediamine is hanging on by only one nitrogen, we would say that the ligand is binding in κ1 mode.
Solved Example
Example: In each of the following cases,
(i) describe the denticity;
(ii) indicate the charge on the ligand and on the metal.
Ans: a) tridentate or κ3; ligand = 0; metal = 1+
b) bidentate or κ2; ligand = 1-; metal = 0
c) bidentate or κ2; ligand = 0; metal = 2+
d) tetradentate or κ4; ligand = 2-; metal = 2+
e) tridentate or κ3; ligand = 0; metal = 1+
f) bidentate or κ2; ligand = 0; metal = 1+
Isomers
Coordination isomers in polydentate ligands
Now, just because a ligand could bind through multiple atoms does not mean that it always binds that way. That's often true with the acetate ligand, for example, because the four-membered ring that forms when it binds through both oxygen atoms is a little too strained. Consequently, there are examples of binding through both oxygen atoms, and there are also examples of binding through only one. Sometimes, acetate uses one oxygen to bind to one metal atom and the other oxygen to bind to a second metal atom, forming a bridge.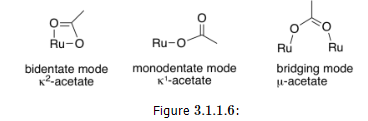
Usually, if a ligand is capable of chelation, assume it binds that way. However, there may be cases in which you are asked specifically to draw it binding in one way or another.
Solved Examples
Example 1: In the following cases, the ligand has slipped so that it isn't binding as tightly as it possibly could. In each case,
(i) describe the denticity as drawn;
(ii) state the maximum denticity possible;
(iii) indicate the charge on the ligand and on the metal.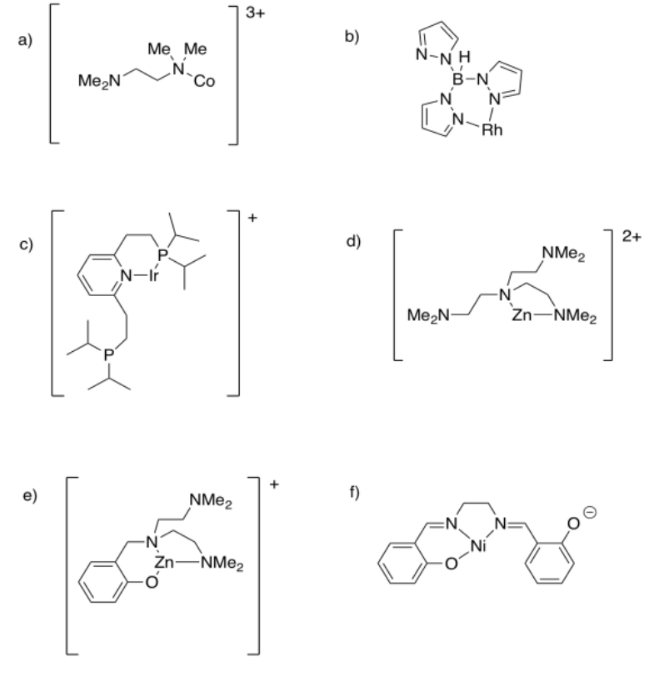
Ans: a) monodentate or κ1; maximum bidentate or κ2; ligand = 0; metal = 2+
b) bidentate or κ2; maximum tridentate or κ3; ligand = 1-; metal = 1+
c) bidentate or κ2; maximum tridentate or κ3; ligand = 0; metal = 1+
d) bidentate or κ2; maximum tetradentate or κ4; ligand = 0; metal = 2+
e) tridentate or κ3; maximum tetradentate or κ4; ligand = 1-; metal = 2+
f) tridentate or κ3; maximum tetradentate or κ4; ligand = 2-; metal = 2+
Example 2: Determine the denticity of each ligand in the following complexes.
a) [Cu(en)2(OH)2]
b) [RuCl2(dmpe)(bpy)]
c) [Ni(acac)2(OH2)2]
d) K2[Cu(ox)2(OH2)2]
e) [FeH2(dmpe)2]
f) [RuH(OAc)(PPh3)3]
g) [Co(en)2Cl2]BF4
h) [Ru(bpy)2(HOCH2CH3)2](ClO4)2
Ans: a) en = bidentate; OH = monodentate
b) dmpe = bidentate; bpy = bidentate; Cl = monodentate
c) acac = bidentate; H2O = monodentate
d) ox = bidentate; H2O = monodentate
e) dmpe = bidentate; H = monodentate
f) H = monodentate; PPh3 = monodentate; OAc = bidentate. In this case, the acetate gets the complex to 18 electrons by binding twice.
g) en = bidentate; Cl = monodentate
h) bpy = bidentate; HOCH2CH3 = monodentate
Stereoisomers in polydentate ligands
Polydentate ligands can give Λ \ Δ isomers and mer\fac isomers, respectively. See the examples below.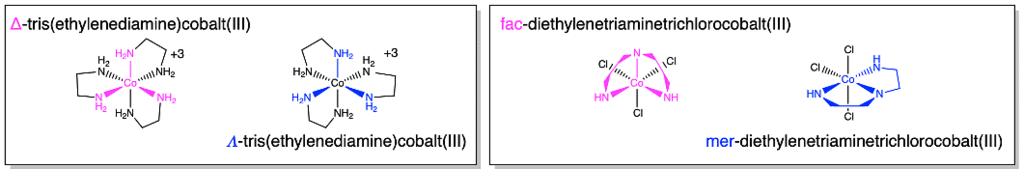
Figure 3.1.1.7: The two molecules shown on the left are Λ and Δ stereoisomers of tris(ethylenediamine)cobalt(III). The two molecules on the right are fac and mer isomers of diethylenetriaminetrichlorocobalt(III).
Bite Angle
- There are more subtle aspects of chelation. For example, two different bidentate ligands may not necessarily bind to the metal in exactly the same way. In the drawing below, it's apparent that the three bidentate phosphine ligands, bis(dimethylphosphino)methane, bis(dimethylphosphino)ethane, and bis(dimethylphosphino)propane, do not all bind the metal with the same geometry. In each case, the metal forms a different angle with the two phosphines.

- The term "bite angle" is frequently used to describe how different bidentate ligands will attach to metals at different angles. In the picture, the P-Pd-P angle appears to be about 90 degrees when dmpm is bound; in reality it is even smaller. With dmpe, the bite angle appears larger in the picture than the one for dmpm, and in reality it is larger, although not quite as large as it appears here.
- Two different ligands that bind with two different bite angles will have different influences on the complex that forms. In fact, chemists often use these differences to "tune" the behavior of transition metals that are used as catalysts for important properties. They might add similar ligands with different bite angles to see which one best promotes the desired catalytic reaction.
- Many factors can influence the bite angle, including structural features of the bidentate ligand itself, the metal, and other ligands bound to the metal. However, a particular ligand will usually have a normal range of bite angles that it will be able to adopt under different circumstances.
Solved Examples
Example 1: Certain ligands may have natural bite angles that work better in some cases than in others. Propose the optimum bite angle in each of the following geometries. Ans:
Ans:



Example 2:
Ans:
The total of the interior angles of a regular polyhedron is given by (n-2)180o, in which n is the number of sides in the polyhedron. Assuming the ring formed by the bidentate ligand and the metal is a regular polyhedron (it won't be, but we are simplifying), then nitrate gives a triangle with 60° angles, including a 60° O-M-O bite angle. Oxalate gives a square with a larger, 90° bite angle.
In reality, the bite angle for nitrate varies with the complex that is formed, but it is usually somewhere around sixty degrees, whereas oxalate usually gives somewhere around eighty five degrees. The smaller ring size gives a smaller bite angle.
Sulfur is larger than oxygen, so its bonds will be a little longer. As a result, you can imagine those two sides of the square being a little longer with sulfur than with oxygen. From the perspective of the metal, the gap between the two donor atoms widens out a little.
Acetate forms bite angles of around sixty degrees, but dithiocarbamate forms larger bite angles of seventy or seventy five degrees.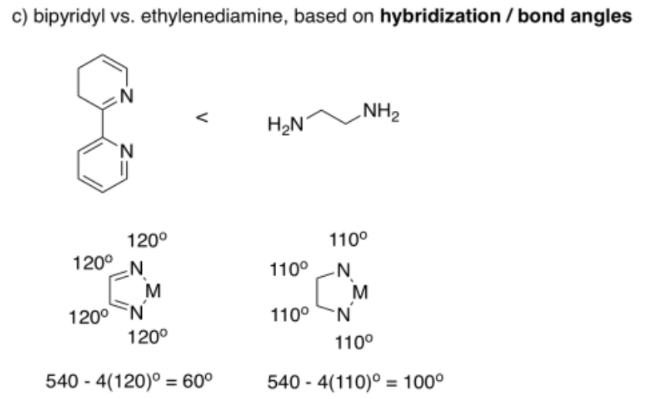
There are lots of differences between these two ligands, but if we simplify and only consider bond angle, we can make a prediction. If the atoms in bipridyl can be considered sp2 hybridised, then they form 120° bond angles. The atoms in ethylenediamine could be considered sp3 hybridised, forming approximately 110° angles. The angle N-M-N still has to complete the shape of the regular pentagon, so if all of the other angles are bigger in the bipyridyl complex, we would expect the bite angle to be smaller.
Really, the bite angles are much closer than this rough estimate suggests. Bipyridyl forms average bite angles of around eighty degrees, whereas ethylenediamine forms average bite angles of around eighty-five degrees. Keep in mind that those are just averages, though. These two values are close enough that their ranges overlap; lots of bipyridyl complexes would have bite angles smaller than ethylenediamine complexes.
The Chelate Effect (and Macrocycle Effect)
Introduction
Monodentate ligands bind through only one donor atom. Monodentate means "one-toothed". The halides, phosphines, ammonia and amines seen previously are monodentate ligands.
Bidentate ligands bind through two donor sites. Bidentate means "two-toothed". An example of a bidentate ligand is bis(dimethylphosphino)propane. It can bind to a metal via two donor atoms at once: it uses one lone pair on each phosphorus atom.
More examples of bidentate ligands are shown below. They all have at least two different atoms with lone pairs. In some cases, there are additional atoms with lone pairs, but only two of them are able to face the metal at one time. Oxalate and glycinate would act as bidentate donors, donating up to two sets of lone pairs at the same time.
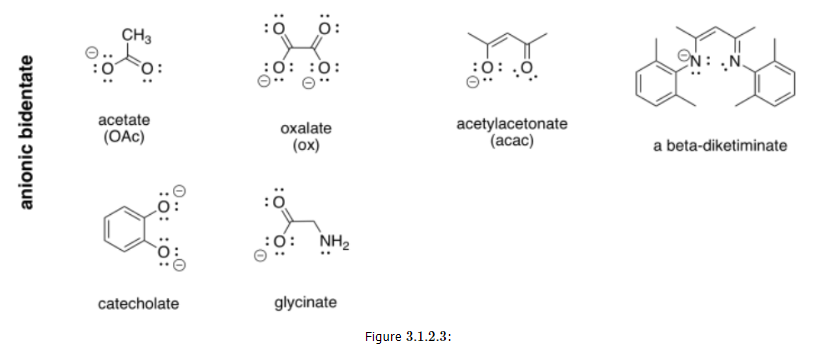
The Chelate Effect
Chelating ligands have higher affinity for a metal ion than analogous monodentate ligands. The chelate effect is the enhanced affinity of a chelating ligand for a metal ion compared to its monodentate ligand counterpart(s). This term comes from the Greek chelos, meaning "crab". A crab does not have any teeth at all, but it does have two claws for tightly holding onto something. A very simple analogy is that, if you are holding something with two hands rather than one, you are not as likely to drop it. For example, ethylenediamine (en, H2NCH2CH2NH2) is a bidentate ligand that binds metal ions more strongly than monodentate amine ligands like ammonia (NH3) and methylamine (CH3NH2). Tridentate ligands, which bind through three donors, can bind even more tightly than bidentate, and so on.
Multidentate ligands bind more tightly because of the chelate effect
Chemical reasoning for the Chelate Effect
The chelate effect can be explained using principles of thermodynamics. Recall that reactions are spontaneous when the Gibbs Free Energy change is negative −ΔG; this is true when change in enthalpy is negative (−ΔH) and the change in entropy is positive (disorder increases, +ΔS. (From the equation ΔG = ΔH − TΔS.)
Consider the reaction shown below: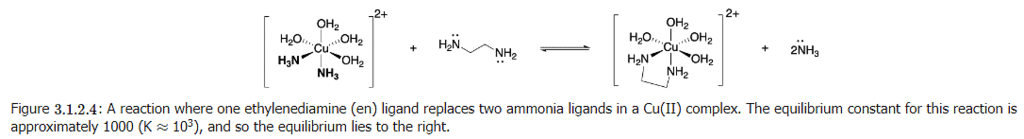
Enthalpy
In each the reactant Cu complex and product Cu-complex in Figure 3.1.2.4, there are two N-Cu bonds. Electronically, the ammonia and en ligands are very similar, since both bind through N and since the Lewis base strengths of their nitrogen atoms are similar. The enthalpy change due to breaking two H3N-Cu bonds and replacing them with two new N(en)-C bonds is almost zero. Thus, enthalpy is not a major driving factor in the chelate effect.
Entropy
In terms of entropy (disorder) there are two things to consider:
- The entropy from free rotation of the chelator. The chelator becomes somewhat constrained upon binding to the metal, and so this would result in small entropic penalty (loss in entropy). This is worth noting, but is a relatively small effect.
- The entropy from change in the number of molecules that can move freely. When a chelating ligand replaces several monodentate ligands, the result is an increase in the number of free molecules in the system, meaning a relatively large increase in entropy. This is the major energetic factor driving the chelate effect.
When a chelating ligand replaces monodentate ligands, there is a relatively large increase in entropy (+ΔS). This is the primary driving factor for the Chelate Effect.
For example, when en replaces two ammonia ligands (Figure 3.1.2.4), the number of total molecules increases from two to three. Increasing the number of molecules by just one is enough to drive the reaction forward.
The example above gives a case when just one bidentate ligand is involved. When multiple bidentate ligands are involved, or when denticity increases, the chelate effect is enhanced further. Consider the two complexation equilibria in aqueous solution, between the cobalt (II) ion, Co2+(aq) and ethylenediamine (en) on the one hand and ammonia, NH3, on the other.
This means that ΔH must be very similar for the two reactions, since six Co-N bonds are formed in each case. Interestingly however, we observe that the equilibrium constant is 100,000 times larger for the second reaction than it is for the first.
The big difference between these two reactions is that the second one involves "condensation" of fewer particles to make the complex. This means that the entropy changes for the two reactions are different. The first reaction has a ΔS value close to zero, because there is the same number of molecules on both sides of the equation. The second one has a positive ΔS° because four molecules come together but seven molecules are produced. The difference between them (ΔΔS) is about +100 J/mol-K. We can translate this into a ratio of equilibrium constants using:
The bottom line is that the chelate effect is entropy-driven. It follows that the more binding groups a ligand contains, the more positive the ΔS° and the higher the Kf will be for complex formation. In this regard, the hexadentate ligand ethylenediamine tetraacetic acid (EDTA) is an optimal ligand for making octahedral complexes because it has six binding groups. In basic solutions where all four of the COOH groups are deprotonated, the chelate effect of the EDTA4- ligand is approximately 1015. This means, for a given metal ion, Kf is 1015 times larger for EDTA4- than it would be for the relevant monodentate ligands at the same concentration. EDTA4- tightly binds essentially any 2+, 3+, or 4+ ion in the periodic table and is a very useful ligand for both analytical applications and separations.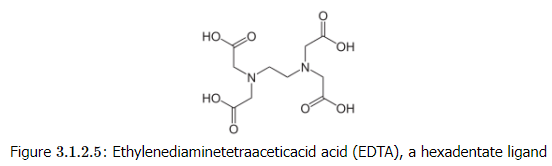
Solved Example
Example: Draw metal complexes using the ligands below, binding to Ni(2+) in a bidentate mode.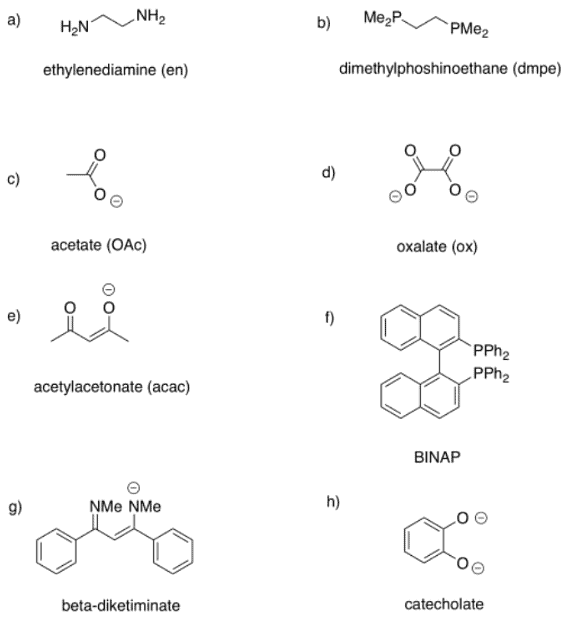
Ans:






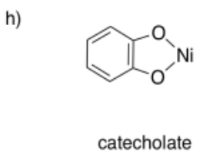
The Macrocycle Effect
The macrocyclic effect follows the same principle as the chelate effect, but the effect is further enhanced by the cyclic conformation of the ligand. Macrocyclic ligands are not only multi-dentate, but because they are covalently constrained to their cyclic form, they also allow less conformational freedom. The ligand is said to be "pre-organized" for binding, and there is little entropy penalty for the ligand to wrap around the metal ion. For example heme b is a tetradentate cyclic ligand which strongly complexes transition metal ions, including (in biological systems) Fe+2.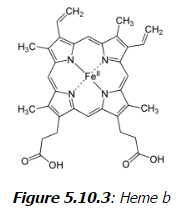
Some other common cyclic ligands are shown below:
- Crown ethers such as 18-crown-6 (below left) are cyclic hard bases that can complex alkali metal cations. Crowns can selectively bind Li+, Na+, or K+ depending on the number of ethylene oxide units in the ring.
- The chelating properties of crown ethers are mimetic of the natural antibiotic valinomycin (below right), which selectively transports K+ ions across bacterial cell membranes and kills the bacterium by dissipating its membrane potential. Like crown ethers, valinomycin is a cyclic hard base.
FAQs on Chelating Ligands and the Chelate Effect - Chemistry Optional Notes for UPSC
| 1. What are chelating ligands and how do they differ from other ligands? |  |
| 2. What is denticity of a ligand and how does it affect coordination isomers? |  |
| 3. What is bite angle and how does it influence the properties of chelate complexes? |  |
| 4. What is the chelate effect and how does it impact the stability of chelate complexes? |  |
| 5. What is the macrocycle effect and how does it relate to chelating ligands? |  |

|
Explore Courses for UPSC exam
|

|


















