Revised National Tuberculosis Control Programme (RNTCP) | Medical Science Optional Notes for UPSC PDF Download
| Table of contents |

|
| RNTCP |

|
| END TB Strategy |

|
| Latent TB-Diagnosis |

|
| Tuberculosis-Treatment |

|
| TB-Causes of Treatment failure |

|
| Drug Resistance in TB (Latest Updates) |

|
| XDR TB |

|
RNTCP
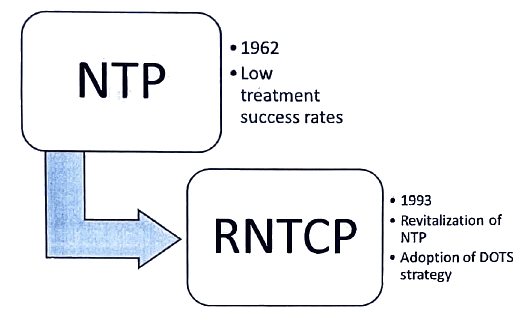
RNTCP-Objectives
- Attainment of a minimum 85 percent cure rate for infectious cases of tuberculosis using Directly Observed Treatment, Short-Course (DOTS) involving peripheral health functionaries.
- Enhancement of case finding activities through high-quality sputum microscopy to identify at least 70 percent of the estimated cases.
RNTCP-Components

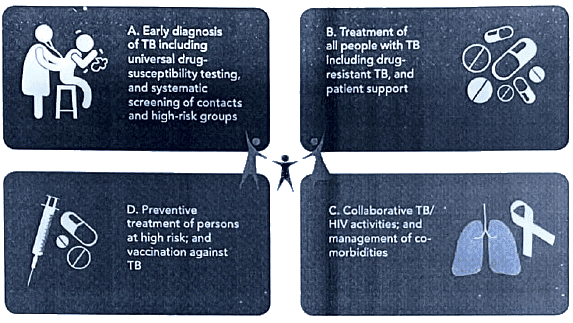
How pillar 1 works : Key components
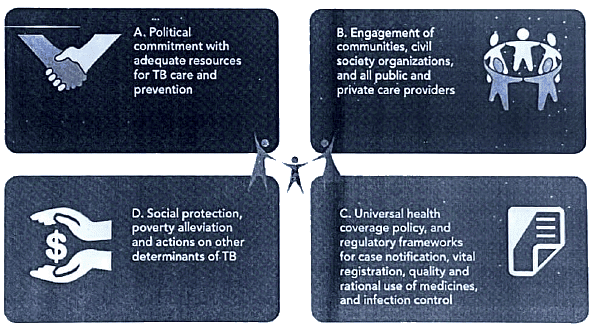
How pillar 2 works : Key components
How pillar 3 works : Key components

END TB Strategy
Aim: To achieve a world without tuberculosis - zero deaths, disease, and suffering due to tuberculosis.
Vision: A world free of tuberculosis with zero deaths, disease, and suffering due to tuberculosis.
Principles:
- Government stewardship and accountability with monitoring and evaluation.
- Forming a robust coalition with civil society organizations and communities.
- Protecting and promoting human rights, ethics, and equity.
- Adapting strategies and targets at the country level with global collaboration.


END TB Strategy- Barriers
The End TB Strategy recognizes four obstacles impeding progress in the battle against TB. They include:
- Fragile health systems
- Root causes of TB such as poverty, undernutrition, migration, an aging population, and risk factors like diabetes, silicosis, and smoking
- Insufficient effective tools
- Persistent unmet funding requirements

per year for implementation of existing TB interventions. An additional gap of USS 1.3 billion exists for research

by health systems every year and therefore may not get adequate care they need

Antiretroviral treatment, treatment of latent TB infection and other key interventions still need further seaie-up

Only one In four MOR-TB cases detected and one in two cases cured
How to overcome barriers/hurdles
Latent TB-Diagnosis
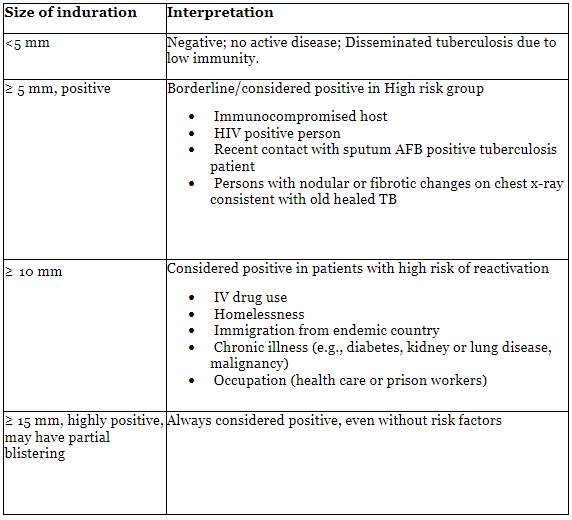
Mantoux test
- Infection with M. tuberculosis elicits a delayed-type hypersensitivity reaction to specific antigenic components of the bacilli. The intradermal administration of the purified protein derivative (PPD) of tuberculin is employed to stimulate a memory T cell response to mycobacterial antigens.
- Procedure: Using a 26-gauge needle and tuberculin syringe, 0.1 ml of PPD is injected intradermally into the volar aspect of the forearm, creating an induration of one-quarter to one-half inch.
- Interpretation: Between forty-eight to seventy-two hours post-injection, the diameter of induration should be measured transversely to the long axis of the forearm and recorded in millimeters.
Tuberculosis-Treatment
Directly Observed Treatment (DOT) is a crucial component of the DOTS strategy, ensuring the administration of the complete treatment course with the right drugs, proper doses, and at the correct intervals.
- In DOT, an observer oversees and supports the patient in taking their medication.
- The health worker or community volunteer responsible for administering DOT is referred to as the 'DOT Provider.'
- DOT can be administered by anyone other than the patient's family members.
- The 'DOT center' is the designated location for providing DOT, conveniently accessible to both the patient and DOT provider.
Traditionally, DOTS Plus refers to DOTS programs that incorporate components for the diagnosis, management, and treatment of MDR-TB. These guidelines advocate for the comprehensive integration of DOTS and DOTS-Plus activities under the Revised National Tuberculosis Control Programme (RNTCP). This approach ensures the correct identification and appropriate management of patients with MDR-TB in line with the guidelines outlined in the Revised National Tuberculosis Control Programme DOTS Plus Guidelines from January 2010.
Components of DOTS Plus
- Persistent political and administrative dedication
- MDR-TB diagnosis using quality-assured culture and drug susceptibility testing (DST)
- Adequate treatment approaches employing second-line drugs within suitable management settings
- Continuous provision of quality-assured anti-TB medications
- A recording and reporting system tailored for DOTS-Plus programs, facilitating the monitoring and evaluation of treatment outcomes.
Patient-wise boxes (PWB)
- Medications are provided in patient-specific boxes (PSB) containing the complete treatment course and packaged in blister packs.
- The PSBs are color-coded to denote the category (Red for CAT I, Blue for CAT II, and Green for CAT III).
- Within each PSB, two pouches are present, one for the intensive phase (A) and one for the continuation phase (B).
- For the intensive phase, each blister pack encompasses medicines for a single dose.
- In the continuation phase, each blister pack holds a one-week supply of medication.
- All doses in the intensive phase are administered under the observation of the DOT provider. If a dose is missed during the intensive phase, the patient is contacted within one day, and the missed dose is given on the following day.
- During the continuation phase, at least the first dose of the week is observed directly, and the subsequent doses for the week are self-administered. On the next scheduled visit, the DOT provider collects the empty blister pack before providing the next weekly dose. The patient is contacted and retrieved for treatment within a week of missing a dose in the continuation phase.
- All empty blister packs (both from the intensive and continuation phases) are kept within the patient-specific boxes (PSB).
Category I (red box)
Indications:
New sputum smear-positive
Seriously ill sputum smear-negative
Seriously ill extra-pulmonary
Regimen:
2(HRZE)3
4(HR)3
Extra-pulmonary TB - seriously ill:
- Meningitis
- Pericarditis
- Peritonitis
- Bilateral or extensive pleural effusion
- Spinal TB with neurological involvement
- Intestinal
- Genitourinary
- Co-infection with HIV
- All forms of pediatric extra-pulmonary TB, excluding lymph node TB and unilateral pleural effusion
Smear-negative pulmonary TB - seriously ill:
- Miliary TB
- Extensive parenchymal infiltration
- Co-infection with HIV
- Cavitations
- All forms of sputum smear-negative pulmonary tuberculosis, except primary complex
Category II (Blue box)
Indications:
- Previously treated sputum smear-positive
- Relapse
- Failure
- Treatment after default
Regimen:
2(HRZES)3
1(HRZE)3
5(HRE)3
Category III (Green box)
Indications:
- New smear-negative and extra-pulmonary, not seriously ill
Regimen:
2(HRZ)3
4(HR)3
Mode of administration and follow up
Mode of Administration:
- Intensive Phase (IP): Three times weekly (Monday, Wednesday, Friday, or Tuesday, Thursday, Saturday), with each dose administered under direct observation.
- Continuation Phase (CP): Three times weekly (Monday, Wednesday, Friday, or Tuesday, Thursday, Saturday), with the first dose of the week administered under direct observation.
Follow-up Sputum Examination Schedule:
- First follow-up: At the end of the intensive phase in all categories.
- Second follow-up: Two months after starting the continuous phase.
- Final follow-up: At the end of the treatment.
TB-Causes of Treatment failure
Doctors - contributing factors to TB drug treatment failure:
- Inappropriate guidelines
- Non-compliance with guidelines
- Absence of guidelines
Drugs - factors leading to inadequate treatment:
- Poor quality
- Irregular supply
- Incorrect delivery (dose/combination)
- Unsuitability due to drug resistance
Patients - factors contributing to TB drug treatment failure:
- Lack of information
- Insufficient funds for treatment and/or transport
- Actual or presumed side effects
- Lack of commitment to a prolonged course of drugs
- Malabsorption
- Social barriers
Category IV (DOTS PLUS)-MDR TB
- Multidrug-resistant tuberculosis (MDR-TB) occurs when the bacilli exhibit resistance to isoniazid and rifampin, which are the most potent bactericidal agents in the treatment of tuberculosis.
- In the management of MDR-TB, new drugs such as Bedaquiline and Delamanid may be employed in cases where an effective WHO-recommended standard MDR-TB regimen cannot be formulated due to known resistance, intolerance, or the unavailability of any second-line drugs in the regimen.

More about Bedaquiline
- Mechanism of action: Bedaquiline specifically targets mycobacterial ATP synthase, an enzyme crucial for supplying energy to the mycobacterium.
- The essential criteria for patient selection for the use of Bedaquiline include:
- The patient's age should be 18 years or older, and they should have MDR TB.
- Pregnancy is an absolute contraindication.
(i) Women should use non-hormonal contraception for birth control throughout the treatment period.
(ii) Women should have been post-menopausal for the past 2 years. - Cardiac arrhythmia is a contraindication due to the risk of QT prolongation.
The following group of people are eligible for use of BDQ therapy
- MDR/RR TB with resistance to any/all FQs or to any/all second-line injectable agents (SLI)
- XDRTB
- Mixed pattern resistant TB (XDR-TB + additional first line / second line resistant TB)
- Treatment failure of MDR-TB + FQ/SLI resistance or XDR-TB
Drug Resistance in TB (Latest Updates)
Resistance can develop to all drugs employed in tuberculosis treatment, resulting in strains of tuberculous bacilli that are not effectively eliminated by the administered anti-tubercular drugs. This resistance can manifest in two distinct types.

Problem Statement
- In 2016, approximately 0.60 million individuals globally were believed to be affected by strains of drug-resistant TB.
- The absence of dependable drug resistance surveillance systems in numerous countries prevents an accurate assessment of drug resistance.
- In India, the estimated prevalence of primary MDR-TB is approximately 2.8%, while in retreatment cases, it ranges from 10-13%.
Causes of Drug Resistant TB (Inadequate treatment)
Poor Treatment, Poor Drugs and Poor Adherence Leads yo Development of MDR TB

Definitions
- Monoresistance: Resistance observed to only a first-line anti-TB drug (e.g., RRTB - Rifampicin-resistant TB).
- Polydrug resistance: Resistance to more than one first-line anti-TB drug (excluding both isoniazid and rifampicin).
- Multidrug resistance (MDR): Resistance manifested against at least both isoniazid and rifampicin.
- Extensive Drug resistance (XDR): Resistance observed against isoniazid and rifampin, along with any fluoroquinolone and at least one of the three injectable second-line drugs (i.e., amikacin, kanamycin, or capreomycin).
Management
The medications suggested for the treatment of patients with MDR/RR TB are categorized into four groups based on their effectiveness, history of use, and drug class.
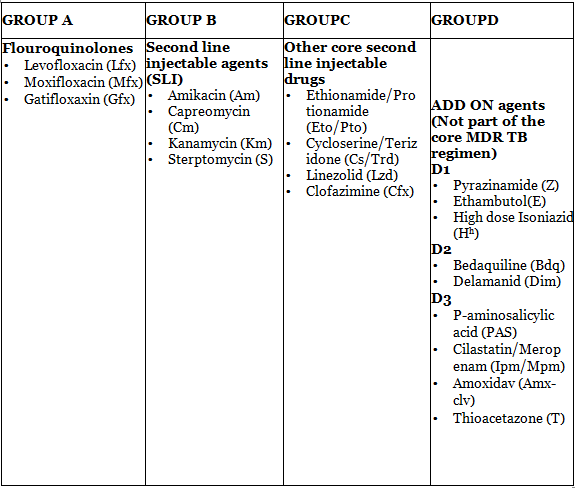
For individuals with RR or MDR TB, it is advisable to employ a treatment plan incorporating a minimum of five potent TB medications during the Intensive phase. This should encompass Pyrazinamide (Z) along with four second-line TB drugs—one from group A, one from group B, and two from group C. In cases of MDR/RR-TB, the regimen should be fortified with either Hh (high dose Isoniazid) or E.
Standard Regimens are

Exclusion criteria for the shorter MDR-TB regimen include:
- Resistance to second-line drugs (fluoroquinolones and/or second-line injectable drugs). Previous exposure exceeding one month to a fluoroquinolone or a second-line injectable medicine not included in the shorter MDR-TB regimen, but potentially leading to cross-resistance, is considered an exclusion criterion. However, if a reliable drug-susceptibility test (DST) confirms the absence of resistance to both of these agents, the shorter MDR-TB regimen may be employed.
- It is not advisable to make treatment decisions based on DST results for any other drug in the regimen (Z, H, E, Eto, Cfz) except those mentioned, due to the unreliable nature of these tests.
- Pregnancy.
- Extra-pulmonary TB, excluding pleural effusion and lymph node TB.
XDR TB
Extensively drug-resistant tuberculosis (XDR TB) is characterized by resistance to isoniazid and rifampin, as well as resistance to any fluoroquinolone and at least one of three injectable second-line drugs (amikacin, kanamycin, or capreomycin). Patients diagnosed with XDR-TB face limited treatment options and a significantly poorer prognosis. Adopting a patient-centric approach, palliative and end-of-life care should be prioritized for these individuals when all available treatment alternatives have been exhausted. Adherence to respiratory infection-control measures is imperative throughout this process.
The US Food and Drug Administration (FDA) has granted approval for a novel oral three-drug regimen designed for extensively drug-resistant tuberculosis (XDR-TB), a condition associated with an estimated 60 percent mortality rate. The treatment regimen consists of pretomanid tablets in combination with bedaquiline and linezolid, collectively referred to as the BPaL regimen, as announced by the FDA on August 14, 2019. This regimen exhibits an efficacy rate of 90 percent and requires administration to patients for only six months.
Latest (Nix TB trial)
Nix-TB stands as a pivotal tuberculosis (TB) trial that evaluates the effectiveness of the three-drug BPaL regimen, comprising bedaquiline, pretomanid, and linezolid, collectively known as the BPaL regimen.
- The trial recruited participants with extensively drug-resistant TB (XDR-TB) at sites in South Africa.
- Inclusivity extended to patients aged 14 and above, including those co-infected with HIV and possessing a CD4 count of 50 or higher.
- The trial encompassed 109 individuals with XDR-TB, along with those exhibiting treatment intolerance or non-responsive multidrug-resistant TB (MDR-TB).
- Data from Nix-TB revealed a favorable outcome in 95 out of the initial 107 patients following six months of treatment with BPaL, accompanied by an additional six months of post-treatment follow-up.
- Nix-TB has successfully completed its enrollment process.
Pretomanid Tablets, in conjunction with bedaquiline and linezolid, received approval from the US FDA on August 14, 2019, for the treatment of a distinct form of extensively treatment-resistant tuberculosis (TB) affecting the lungs.
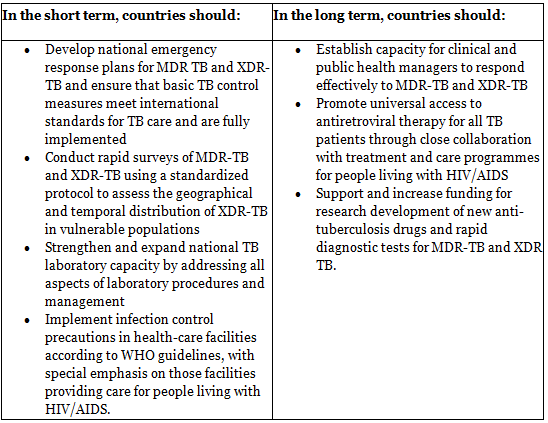
7 Point plan of action (Steps to limit the impact of MDR TB and XDR TB)
The rise of extensively drug-resistant tuberculosis (XDR-TB) and the increased mortality rate in individuals with HIV infection were discussed during emergency consultations held in Johannesburg on 7-8 September 2006.
The agenda encompassed enhancing treatment adherence to attain high completion levels (> 85 per cent) for all TB patients and ensuring strict control and proper utilization of second-line drugs for MDR-TB and XDR-TB in accordance with WHO guidelines.
The measures needed to mitigate the impact of MDR-TB and XDR-TB were identified and integrated into a 7-point action plan.
Preventing Drug Resistance: Given that incomplete, inadequate, and irregular treatment is the primary factor leading to drug resistance, prevention measures include:
(a) Administering treatment with a combination of two or more drugs
(b) Utilizing drugs to which the bacteria are susceptible
(c) Ensuring that the treatment is comprehensive, sufficient, and consistent.
RNTCP-Repeats
Tuberculosis
- Drug resistant tuberculosis has emerged as a major public health challenge before the country. Keeping this in context, answer the following questions. (2019)
(i) What are the major culpable factors which have led to this situation?
(ii) To limit the burden of multi-drug resistant tuberculosis (MDR-TB) and extensively drug resistanttuberculosis (XDR-TB) in the community, public health experts have drawn a 7-point plan of action. State those 7 points in brief.
(iii) List the subgroups of tuberculosis patients currently eligible for receiving treatment with a Bedaquiline containing regimen. - The End TB Strategy' is a logical evolution and a paradigm shift from the past global TB strategies. (2017)
(i) What are its vision, goal, milestones and target indicators that are set to be achieved in specific future years?
(ii) What are the major hurdles which stand in its success?
(iii) What steps are required to overcome these hurdles? - Write briefly on Stop 'TB Strategy'. (2016)
- List the components of the revised national tuberculosis control programme. How is tuberculin test interpreted? Analyze the reasons for high defaulter rates in the treatment of pulmonary tuberculosis (2004).
- Write about Revised National Tuberculosis Control Programme. (2011)
- Directly observed Treatment Short Course Chemotherapy (DOTS) in Pulmonary Tuberculosis Control Programme (2001)
|
7 videos|219 docs
|
FAQs on Revised National Tuberculosis Control Programme (RNTCP) - Medical Science Optional Notes for UPSC
| 1. What is RNTCP and how does it contribute to the END TB Strategy? |  |
| 2. How is latent TB diagnosed? |  |
| 3. What is the treatment for tuberculosis? |  |
| 4. What are the causes of treatment failure in tuberculosis? |  |
| 5. What are the latest updates on drug resistance in TB? |  |

|
Explore Courses for UPSC exam
|

|
















