UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > The 18 Electron Rule
The 18 Electron Rule | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Electron Counting In Transition Metal Complexes |

|
| The Neutral Atom Method |

|
| "Oxidation State" Method |

|
| Counting Electrons: Ligand Contributions |

|
| 18 Electron Rule |

|
Electron Counting In Transition Metal Complexes
- In this chapter we will learn how to count valence electrons in coordination compounds. Electron counting is important because the number of electrons in a complex can tell us a lot about the stability and reactivity in a coordination compound. In addition, it allows us to predict and understand structures to a certain extent.
- Electron counting sounds trivial, but it is not as trivial as it seems, actually there are even two different methods for electron counting. Each method leads to the same result. Which method you prefer is “personal taste”, but each method is about equally common in the literature, so you need to know both of them.
The Neutral Atom Method
- The first method is called the “neutral atom method”. As the name suggests, we will break up the complex into neutral fragments, and count the electrons that contribute to the bonding in each of the fragments. The neutral atom method is carried out according to the following three steps. First, we count the number of the valence electron of the metal. We consider the metal as neutral atom. The number valence electrons is the same as the group number of the transition metal in the periodic table. For transition metals the group number varies from 3 to 12. In the second step, we account for the ionic charge of the complex, if the complex is not neutral.
- This will reduce the number of electrons for a complex cation, and increase the number of valence electrons for a complex anion. In the third step, we need to determine how many electrons are contributed by each ligand. This is the most complicated step. To determine the number of electrons must cleave each metal-ligand bond so that a ligand fragment results that is neutral. We then count the number of electrons at the ligand that contributed to the bond. The number of electrons contributed by each ligand is then summed up. This is sum is then added to the number of electrons determined by the previous steps. This is gives the overall number of valence electrons.

- Let us apply these rules by an example, the cis-platinum complex. We first need to count the number of electrons of the metal. The metal is platinum which is located in group 10 of the periodic table. Therefore, a neutral platinum atom has ten valence electrons.
- Next, we look at the charge of the complex. In this case, there is no charge, and therefore no electrons are added or subtracted.
- Lastly, we count the electrons of the ligands. There are two types of ligands, the chloro-ligands and the ammine ligands. Now, our task is to cleave the Pt-ligand bond so that neutral ligand fragments result. We can see that for the chloro-ligands we must cleave the Pt-Cl bond, homoleptically, meaning in the middle, assigning one electron to the Pt and one electron to Cl, because doing so creates a neutral chlorine atom. The fact that we cleaved the bond homoleptically, means that the chloro ligand contributed one electron. Because we have two chloro ligands, there are overall two electrons.
- Now, let us think about how many electrons the ammine ligands contribute. In this case we need to cleave the metal-ligand bond heteroleptically to produce a neutral ligand fragment. Both bonding electrons are assigned to the ligand. This produces a neutral NH3 molecule. This means that each ammine ligand contributes two electrons. Overall, that makes four electrons, because we have two ammine ligands.
- Finally, we need to sum up the electrons from all three steps. That is ten electrons from Pt, zero electrons due to charge, two electrons from the chloro ligands, and four electrons from the ammine ligands equaling 16 valence electrons total. This is the final result.
Question for The 18 Electron RuleTry yourself: What is the total number of valence electrons in the cis-platinum complex?View Solution
"Oxidation State" Method
- The oxidation state method is also comprised of three steps. The first step is the same as in the neutral atom method. We determine the number of electrons of the neutral metal which the same as its group number in the periodic table. The next step is different though. It requires the determination of the oxidation state of the metal. How can we determine it? First, we cleave the metal-ligand bonds heteroleptically so that all bonding electrons are assigned to the ligands. Then we determine what is the charge of the ligands? Ligands can either be neutral of negatively charged. We determine the overall number of charges at the ligands.
- The difference between that number and the charge of the complex is the oxidation state of the metal. We either add or subtract electrons depending on the oxidation state of the metal. If the oxidation state is positive we subtract electrons, if it is negative, which is rare, then we add electrons. The third step counts the number of the electrons contributed by the ligands. Because we cleaved all bonds homoleptically, all bonding electrons are considered to be contributed by the ligands, and we count them accordingly. Finally, we sum up the electrons of all three steps which gives the total number of electrons.

- Let us apply the method to the previous example cis-platinum. Applying the first step gives us 10 valence electrons for the platinum.
- Next, we need to determine the oxidation state of Pt. To do so, we must now cleave all metal-ligands heteroleptically, so that all bonding electrons are assigned to the ligand. By the way, this is equivalent to saying that we treat all the bonds as dative bonds with all electrons coming from the ligands as the donors. When we do this for the chloro ligands we see that this created chloride anions with a 1- charge. Cleaving the Pt-N bonds heteroleptically leads to neutral NH3 molecules. Therefore the overall number of charges at the ligands is 2x(-1)+2x0=-2. The charge at the complex is zero, therefore the oxidation state of Pt is 0-(-2)=+2. We must therefore subtract two electrons from the 10 electrons of the platinum.
- Now we must determine the number electrons coming from the ligands. Because all bonds are considered as dative bonds, the chloro ligands contribute two electrons each, and the ammine ligands contribute two electrons each. That makes overall four electrons.
- In sum, 10 electrons from the neutral Pt atom minus two electrons due to the +2 oxidation state of Pt plus 2x2=4 electrons from Cl plus 2x2=4 electrons from NH3 gives 16 electrons total. We can see that we have arrived at the same results as in the case of the oxidation state method.
- We can discuss the advantages and disadvantages of both methods, also. They neutral atom method has the advantage that we do not have to think about charges at ligands and oxidation states. However, we have to think about how to cleave bonds to create neutral fragments. We may need ot cleave bonds in an way that is not reflecting the donor-acceptor nature of a coordination compound. The oxidation method does account for the donor-acceptor nature of a coordination compound because the bonds are considered dative bonds, and the electrons are assigned to the ligands and metal accordingly. We not not need to think how to cleave bonds, because we cleave the bonds always heteroleptically. However, it requires us to think about charges at the ligands to determine oxidation states which is an additional, non-trivial step.
Counting Electrons: Ligand Contributions
- The most difficult step in electron counting is mostly the determination of the number of electrons a ligand provides. Therefore, let us practice this by a few example.

- Let us first look at an hydrido ligand which for example occurs in the nonohydridorhentate(2-) complex. What is the charge of the ligand in the two methods? In the neutral atom method the charge is always zero, this is a no-brainer. In the case of the oxidation statement method, we need to treat the bond as a dative bond, and that means that we must cleave the bond heteroleptically, so that both bonding electrons can be assigned to the ligand.
- An H atom with two electrons is a hydride anion with a -1 charge. Next, let us think about the number of electrons donated. In the neutral atom method we need to produce neutral ligand fragments. To do so we must cleave the M-H bond homoleptically, because this will create a neutral hydrogen atom. How many electron will it contribute. It will contribute one electron, because we cleaved the bond homoleptically assigning only one of the two bonding electrons to H. In the oxidation state method, the hydrido ligand contributes two electrons because the bond was considered dative and therefore cleaved heteroleptically. Both bonding electrons were assigned to the ligand, therefore the ligand contributes two electrons.
 Next, let us consider a halogenide ligand. What is the charge at the ligand. For the neutral atom method, the answer is trivial, the charge is always zero. In the oxidation state method both bonding electrons in the M-L bond get assigned to the ligand. This gives the ligand a -1 charge. What is the number of electrons contributed? In the neutral atom method we need to think again how to cleave the M-L bond to create a neutral ligand fragment. We need to see that we must cleave the bond homoleptically to produce that fragment. Cleaving the bond homolopetically means that that ligand has contributed one electron. In the oxidation state method, we cleave the bond always heteroleptically so that all bonding electrons are assigned to the ligand. Thus, the ligand contributes two electrons.
Next, let us consider a halogenide ligand. What is the charge at the ligand. For the neutral atom method, the answer is trivial, the charge is always zero. In the oxidation state method both bonding electrons in the M-L bond get assigned to the ligand. This gives the ligand a -1 charge. What is the number of electrons contributed? In the neutral atom method we need to think again how to cleave the M-L bond to create a neutral ligand fragment. We need to see that we must cleave the bond homoleptically to produce that fragment. Cleaving the bond homolopetically means that that ligand has contributed one electron. In the oxidation state method, we cleave the bond always heteroleptically so that all bonding electrons are assigned to the ligand. Thus, the ligand contributes two electrons.
- A halogenide anion as a ligand cannot only be terminal, but bridging. An example is the μ-dichloro bis(tetraethylene rhodium(I)) complex in which two chloro-ligands bridge two rhodium atoms. What is the charge in the neutral atom method. Of course, it is zero. What is the charge in the oxidation state method? We can see that if we consider both metal-ligand bonds dative bonds, and cleave the bonds heteroleptically, that the Cl atom is surrounded by eight unshared electrons, which give it a -1 charge. How, many electrons are contributed in the neutral atom method? To answer this question, we need to decide if we have to cleave the bonds homo- or heteroleptically to produce a neutral Cl atom. Can you see it? The answer is: We must cleave one bond homoleptically, and the other one heteroleptically. How many electrons are then contributed by the chloro ligan? It is the two electrons from the heteroleptically cleaved bond, and one electron from the homoleptically cleaved bond. So overall it is three electrons. What about the oxidation state method? In this case both bonds very cleaved heteroleptically, and this means that overall four electrons have been contributed.

- What are the charges and electrons contributed by an alkoxy ligand? An example for a complex with such as ligand is pentaphenoxy ferrate (3-). The charge according to the neutral atom method is zero. In the oxidation state method we cleave the bonds heteroleptically, and our ligand becomes an alkoxide anion. This anion has a 1- charge. How many electrons does the ligand contribute? To get a neutral fragment we must cleave the bond homoleptically. This actually produces an alkoxy radical. This radical contributes its radical electron, thus there is one contributed electron. In the oxidation state method we treated the bond as a dative bond and cleaved the bond heteroleptically. Therefore two electrons are contributed according to the oxidation state method.

- Next, let us apply electron counting to the carbonyl ligand. For example four carbon monoxide molecules form an iron pentacarbonyl complex with iron. The charge at the ligand in the neutral atom method is zero. In the oxidation state method, we assign both bonding electrons to the ligand, and that produces a neutral carbon monoxide molecule. Note that the carbon atom is formally negatively charged, because it is surrounded by 5 electrons, but the oxygen atom is positively charged because it is surrounded by five electrons. So overall, the molecule is neutral. What is the number of electrons contributed in the neutral atom method? To produce a neutral ligand we must cleave the bond heteroleptically. This produces a neutral carbon monoxide molecule. Because we cleaved the bond heteroleptically, the ligand contributes two electrons. In the oxidation state method we always cleave the bonds heteroleptically, and thus two electrons come from the ligand.

- The last ligand we discuss here is the isonitrile ligand. An example is the pentakis-(tert-butyl isonitrile) iron molecule. The charge of the ligand is zero in the neutral atom method, but what is it in the oxidation state method? Let us see what happens as we assign both bonding electrons to the ligand. We we see that the carbon is now surrounded by five electrons. Three are in the carbon-nitrogen triple bond and they other two come from the electron lone pair at the carbon atom. This means that the carbon atom has a -1 charge. Now, let us look at the N atom. We see that it is surrounded by four electrons, three coming from the C-N triple bond, and one from the C-R bond. That means that the N atom has a +1 charge. Overall, the molecule is neutral and does not carry a charge. What about the number of electrons contributed? We can see that we must cleave the bond heteroleptically to produce a neutral isoitrile, there are two electrons are contributed in the neutral atom method. The oxidation state method must cleave the bond heteroleptically, therefore, the number of electrons is also two.
Question for The 18 Electron RuleTry yourself: What is the 18 electron rule in coordination chemistry?View Solution
18 Electron Rule
- Electron counting is important in the context of an important rule in coordination chemistry: The 18 electron rule. The 18 electron rule states that for d-block elements normally complexes with 18 electrons in the shell (ns2(n-1)d10np6 configuration) are most stable. If this number is not reached, the species is coordinatively unsaturated and tend to add more ligands. It also tends to be reduced because adding electrons brings the complex to or at least closer to 18 electrons. Coordinatively unsaturated complexes therefore tend to have a higher reactivity.
- Definition: Coordinatively Unsaturated: A species is coordinatively unsaturated when the 18 electrons are not reached in the (ns2(n-1)d10np6 configuration) shell. They tend to add more ligands, and to be reduced. They are associated with higher reactivity.
- If a species has more than 18 electrons it is coordinatively oversaturated and tends to lose ligands. It is usually easily oxidized. Both loss of ligands and oxidation reduces to the number of electrons to or at least closer to 18.
- Definition: Coordinatively Oversaturated: A species is coordinatively oversaturated when it has more than 18 electrons in the shell (ns2(n-1)d10np6 configuration) shell. They tend to lose ligands and are easily oxidized.
- The 18 electron rule has many exceptions, and therefore needs to be applied with caution. In particular group 3, 4, and 10 complexes deviate often from the 18 electron rule.

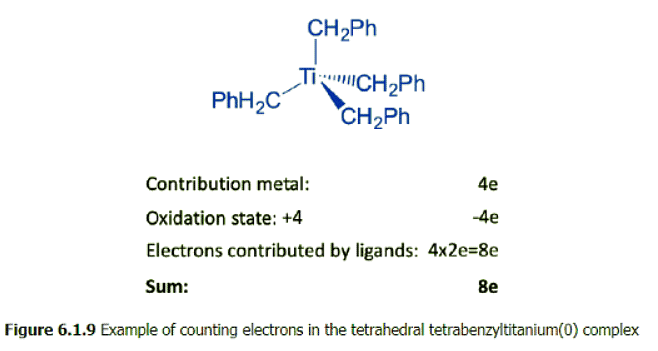
- For illustration purposes, let count the number of electrons of the tetrahedral tetrabenzyltitanium(0) complex by the oxidation state method. We could also use the neutral atom method, which would give the same results. This complex is a group 4 complex because titanium is in group 4. How many electrons will the titanium contribute? Because number of electrons is always the same as the group number, it will contribute four electrons. Next, what is oxidation state of Ti? To determine it we must determine the charge at the ligands. To do that we cleave the bonds heteroleptically.
- This will give benzylate anions with -1 charge. There are four of these ions, and therefore there will be four negative charges overall. The complex is charge-neutral, and thus the oxidation state is +4 because -4+4=0. Therefore, we need to subtract four electrons. Because we cleaved the bond heteroleptically, each ligand contributed two electrons, giving overall eight electrons coming from the four ligands. This means that we have overall eight electrons, or an 8-electron complex. This is far, far away from 18 electrons. Nonetheless, the complex is quite stable, does not have a tendency to get reduced, or to add ligands. How can we explain this.
- The answer is that in order to achieve 18 electrons it would need to add five additional ligands if each ligand is considered a 2-electron donor. This would increase the coordination number to 9 which is too high to produce a stable complex. In order to reduce the complex to an 18 electron complex, 10 electrons would need to be added. This would produce a complex with a -10 charge which is way to high to be stable. The arguments are generalizable for group 3 and group 4 complexes. Because these elements only have a few d electrons, the ligands would contribute a lot of electrons to produce an 18 electrons complex. This would require just too many ligands to add. The coordination numbers would get too high. If electrons are added instead to ligands, the negative charge at the complex would be to high to be stable based on electron-electron repulsion arguments.

- These arguments cannot be applied for group 10 elements, because these elements have many d electrons. The explanation in this case is that these elements like to make square planar complexes when in the common oxidation state +2. Square planar complexes prefer 16 instead of electrons. You can see that the square planar dichlorodiammine palladium complex shown is square planar and has sixteen electrons. There are 10 electrons coming from Pd. If we use the neutral atom method, no electrons need to be added or subtracted due to the charge at complex. The complex is charge-neutral. To assess how many electrons come from the ligands we need to cleave the bonds so that neutral ligands are produced. The Pd-Cl bonds need to be cleave homoleptically, the Pd-N bonds need to be cleave heteroleptically. Therefore, the two chloro ligands are 1e donors, and the two ammine ligands are 2e donors. This gives 10+4+2=16 electrons
The document The 18 Electron Rule | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on The 18 Electron Rule - Chemistry Optional Notes for UPSC
| 1. What is electron counting in transition metal complexes? |  |
Ans. Electron counting in transition metal complexes refers to a method used to determine the number of electrons in the metal center and its ligands. It helps in understanding the overall electronic structure and reactivity of the complex.
| 2. What is the Neutral Atom Method of electron counting? |  |
Ans. The Neutral Atom Method is a way of counting electrons in transition metal complexes. In this method, the metal center is considered as a neutral atom and the number of electrons is counted based on its position in the periodic table. For example, a transition metal in Group 8 of the periodic table is considered to have 8 valence electrons.
| 3. What is the "Oxidation State" Method of electron counting? |  |
Ans. The "Oxidation State" Method is another approach to electron counting in transition metal complexes. In this method, the metal center is assigned an oxidation state based on its charge in the complex. The number of electrons contributed by the metal center is then determined based on its oxidation state and the number of valence electrons it would have in that oxidation state.
| 4. How do ligands contribute to electron counting in transition metal complexes? |  |
Ans. Ligands are molecules or ions that bind to the metal center in a transition metal complex. They contribute electrons to the overall electron count of the complex. The number of electrons contributed by the ligands depends on their charge and the number of lone pairs they possess. Typically, each negatively charged ligand contributes two electrons, while neutral ligands contribute zero electrons.
| 5. What is the 18 Electron Rule in electron counting? |  |
Ans. The 18 Electron Rule is a guideline that suggests stable transition metal complexes often have 18 valence electrons in their metal center and ligands combined. This rule helps in predicting the stability and reactivity of transition metal complexes. However, it is important to note that not all complexes strictly follow the 18 Electron Rule and exceptions can exist based on the nature of ligands and coordination geometry.

|
Explore Courses for UPSC exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches



 Next, let us consider a halogenide ligand. What is the charge at the ligand. For the neutral atom method, the answer is trivial, the charge is always zero. In the oxidation state method both bonding electrons in the M-L bond get assigned to the ligand. This gives the ligand a -1 charge. What is the number of electrons contributed? In the neutral atom method we need to think again how to cleave the M-L bond to create a neutral ligand fragment. We need to see that we must cleave the bond homoleptically to produce that fragment. Cleaving the bond homolopetically means that that ligand has contributed one electron. In the oxidation state method, we cleave the bond always heteroleptically so that all bonding electrons are assigned to the ligand. Thus, the ligand contributes two electrons.
Next, let us consider a halogenide ligand. What is the charge at the ligand. For the neutral atom method, the answer is trivial, the charge is always zero. In the oxidation state method both bonding electrons in the M-L bond get assigned to the ligand. This gives the ligand a -1 charge. What is the number of electrons contributed? In the neutral atom method we need to think again how to cleave the M-L bond to create a neutral ligand fragment. We need to see that we must cleave the bond homoleptically to produce that fragment. Cleaving the bond homolopetically means that that ligand has contributed one electron. In the oxidation state method, we cleave the bond always heteroleptically so that all bonding electrons are assigned to the ligand. Thus, the ligand contributes two electrons.