Oxidation of Alcohols: CrO3, PCC, DMP | Chemistry Optional Notes for UPSC PDF Download
Introduction
The reading mentions that pyridinium chlorochromate (PCC) is a milder version of chromic acid that is suitable for converting a primary alcohol into an aldehyde without oxidizing it all the way to a carboxylic acid. This reagent is being replaced in laboratories by Dess‑Martin periodinane (DMP), which has several practical advantages over PCC, such as producing higher yields and requiring less rigorous reaction conditions. DMP is named after Daniel Dess and James Martin, who developed it in 1983.
Oxidation States of Carbon
The general idea of oxidation and reduction reactions learned in general chemistry is that when a compound or atom is oxidized it loses electrons, and when it is reduced it gains electrons. In order, to keep track of electrons in organic molecules a oxidation state formalism is used. Oxidation states to not represent the actual charge but it will allow the number of electrons being gained or lost by a particular atom during a reaction.
To calculate the oxidation state of a carbon atom the following rules are used:
- A C-C bond does not affect the oxidation state of a carbon. So a carbon attached to 4 carbons has an oxidation state of zero.
- Every C-H bond will decrease the oxidation state of the carbon by 1.
- Each C-X bond will increase the oxidation state of the carbon by 1. Where X is an electronegative, such as nitrogen, oxygen, sulfur, or a halogen.
When looking at the oxidation states of carbon in the common functional groups shown below it can be said that carbon loses electron density as it becomes more oxidized.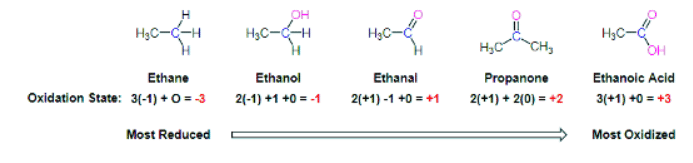 For this section, a simpler way to consider this process is to say that when a carbon atom in an organic compound loses a bond to hydrogen and gains a new bond to a oxygen it has been oxidized. A very commonly example is the oxidation of an alcohol to a ketone or aldehyde. Notice that during this process the carbon atom loses a hydrogen and gains a bond to oxygen.
For this section, a simpler way to consider this process is to say that when a carbon atom in an organic compound loses a bond to hydrogen and gains a new bond to a oxygen it has been oxidized. A very commonly example is the oxidation of an alcohol to a ketone or aldehyde. Notice that during this process the carbon atom loses a hydrogen and gains a bond to oxygen.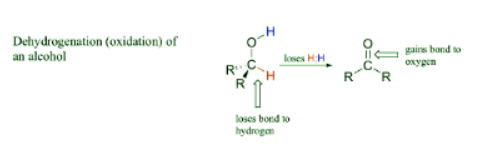
Oxidation of Alcohols
On of the most important reactions of alcohols is their oxidation to carbonyl containing compounds such as aldehyde, ketones, and carboxylic acid. Typically primary alcohols, depending on the reagent used, produce aldehydes or carboxylic acids during oxidations. Secondary alcohols are oxidized to produce ketones, and tertiary alcohols are usually not affected by oxidations.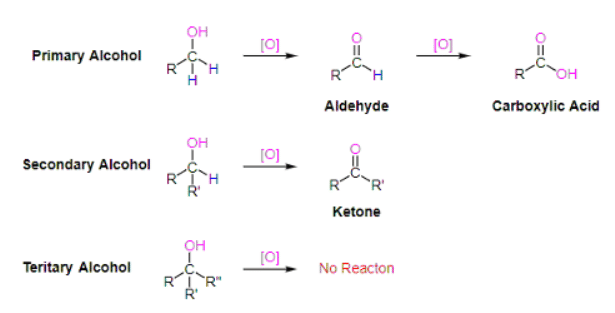
Alcohol Oxidizing Agents
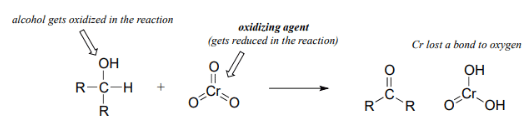
- Oxidation and reduction reactions always occurs in tandem: when one compound is oxidized, another compound must be reduced. For an alcohol to be oxidized in a reaction there must also be a compound being reduced. This reduced compound is also called the oxidizing agent.
- For example, chromium trioxide (CrO3) is a common oxidizing agent used by organic chemists to oxidize a secondary alcohol to a ketone. During this reaction CrO3 is being reduced to form H2CrO3. A common method for oxidizing secondary alcohols to ketones uses chromic acid (H2CrO4) as the oxidizing agent. Chromic acid, also known as Jones reagent, is prepared by adding chromium trioxide (CrO3) to aqueous sulfuric acid.

- There is a wide selection of oxidizing agents available for use in the organic chemistry laboratory, each with its own particular properties and uses. In addition to CrO3, other commonly used oxidizing agents include potassium permanganate (KMnO4) and sodium dichromate (Na2Cr2O7). Any of these reagents can be used to oxidize secondary alcohols to form ketones and primary alcohols to form carboxylic acids. Tertiary alcohols remain unreactive to oxidation.
General Reactions
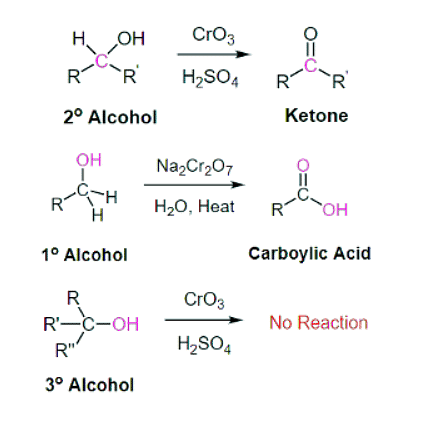
Mechanism
During this reaction mechanism the chromium atom is being reduced from Cr(VI) in the CrO3 starting material to Cr(IV) in the H2CrO3 product. Also, notice the the C=O bond is formed in the third step of the mechanism through an E2 reaction. Although E2 reaction are generally know for forming C=C double bonds thought the elimination of a halide leaving group, in this case they are use to generate a C=O through the elimination of a reduced metal as a leaving group.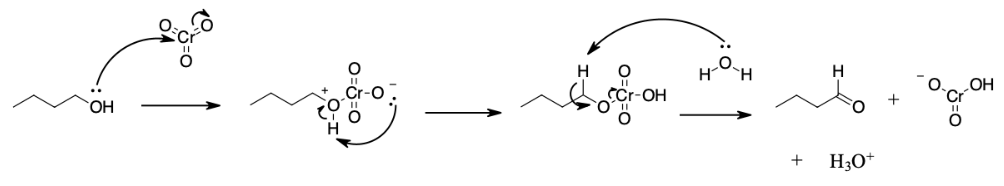
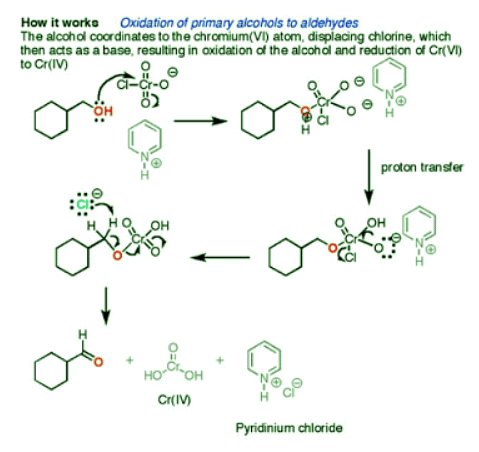
Oxidation of 1° Alcohols with PCC to form Aldehydes
Pyridinium chlorochromate (PCC) is a milder version of chromic acid. PCC oxidizes 1o alcohols one rung up the oxidation ladder, turning primary alcohols into aldehydes and secondary alcohols into ketones. Unlike chromic acid, PCC will not oxidize aldehydes to carboxylic acids. Cr(IV) as well as pyridinium chloride are produced as byproducts of this reaction.
General Reactions
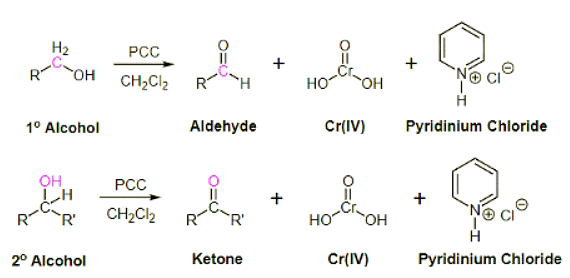
Mechanism
- The first step of the mechanism is attack of alcohol oxygen on the chromium atom to form the Cr-O bond. Secondly, a proton on the (now positive) OH is transferred to one of the oxygens of the chromium, possibly through the intermediacy of the pyridinium salt. A chloride ion is then displaced, in a reaction reminiscent of a 1,2 elimination reaction, to form what is known as a chromate ester.
- The C-O double bond is formed when a base removes the proton on the carbon adjacent to the oxygen. It is also possible for pyridine to be used as the base here, although only very low concentrations of the deprotonated form will be present under these acidic conditions. In an E2 reaction, the electrons from the C-H bond move to form the C=O bond, and in the process break the O-Cr bond. During this step Cr(VI) gains two electrons to become Cr(IV) (drawn here as O=Cr(OH)2).
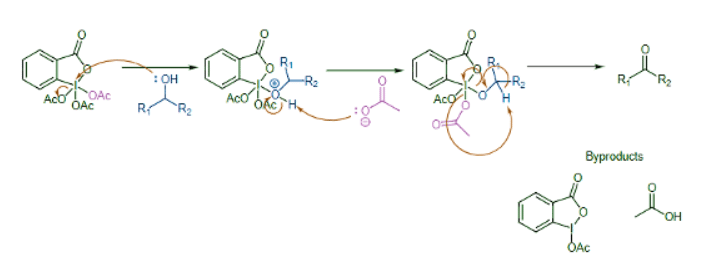
Oxidation of 1º Alcohols with Dess‑Martin Periodinane (DMP) to form Aldehydes
PCC is being replaced in laboratories by Dess‑Martin periodinane (DMP) in dichloromethane solvent, which has several practical advantages over PCC, such as producing higher yields and requiring less rigorous conditions (lower reaction temperature and a nonacidic medium). DMP is named after Daniel Dess and James Martin, who developed it in 1983.
General Reactions

Mechanism
The first step of the mechanism involves the reactant alcohol attacking the Iodine (V) atom and eliminating an acetate (Ac-) leaving group to form a periodinate intermediate. The next step is a concerted E2-like reaction where a hydrogen is removed from the alcohol, the C=O bond is formed, an acetate group is eliminated from the iodine atom, and the iodine (V) atom gains two electrons to be reduced to iodine (III).
Biological Alcohol Oxidations
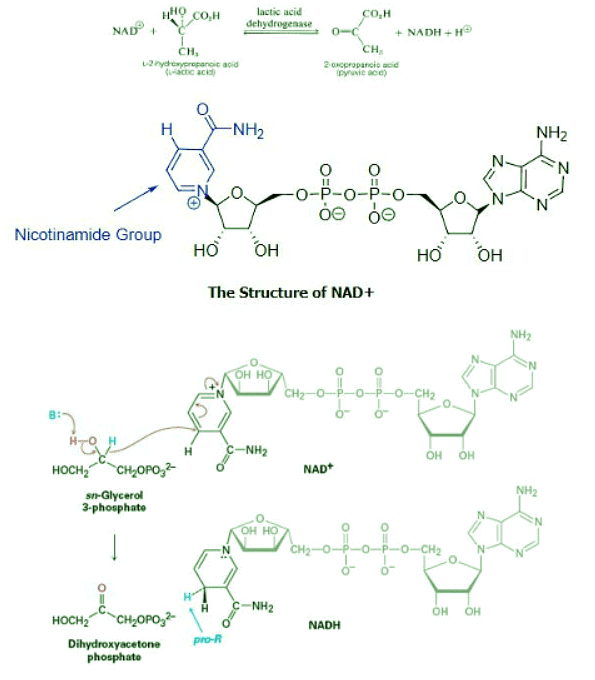
- There are many biological oxidations that convert a primary or secondary alcohol to a carbonyl compound. These reactions cannot possibly involve the extreme pH conditions and vigorous inorganic oxidants used in typical laboratory oxidations. Rather, they occur at nearly neutral pH values and they all require enzymes as catalysts, which for these reactions usually are called dehydrogenases.
- An important group of biological oxidizing agents includes the pyridine nucleotides, of which nicotinamide adenine dinucleotide (NAD+) is an example. This very complex molecule functions to accept hydride (H:-) or the equivalent (H++ 2e−) from the α carbon of an alcohol. The reduced form of NAD+ is abbreviated as NADH and the H:- is added at the 4-position of the pyridine ring. It is important to note that the hydride adds exclusively to the Re face of the pyridine ring giving NADH a pro-R stereochemistry.
- An example of the remarkable specificity of this kind of redox system. One of the last steps in the metabolic breakdown of glucose is the reduction of 2-oxopropanoic (pyruvic) acid to L-2-hydroxypropanoic (lactic) acid. The reverse process is oxidation of L-lactic acid. The enzyme lactic acid dehydrogenase catalyses this reaction, and it functions only with the L-enantiomer of lactic acid. During this reaction a base removes the alcohol hydrogen. The resulting alkoxide ion then forms the C=O bond causing a hydride ion to transfer to NAD+.

Another example is provided by one of the steps in metabolism by way of the Krebs citric acid cycle, is the oxidation of L-2-hydroxy-butanedioic (L-malic) acid to 2-oxobutanedioic (oxaloacetic) acid. This enzyme functions only with L-malic acid:

Solved Examples
Example 1: Show the products of the oxidation of 1-propanol and 2-propanol with chromic acid in aqueous solution.
Ans:
Example 2: Show the products of the oxidation of 1-propanol and 2-propanol with Dess-Martin periodinane.
Ans:
FAQs on Oxidation of Alcohols: CrO3, PCC, DMP - Chemistry Optional Notes for UPSC
| 1. What are oxidation states of carbon? |  |
| 2. How does oxidation of alcohols occur? |  |
| 3. What are alcohol oxidizing agents? |  |
| 4. How is PCC used to oxidize 1° alcohols to form aldehydes? |  |
| 5. What are biological alcohol oxidations? |  |