Olympiad Notes: Changes Around Us - Class 6 PDF Download
Introduction
- Transformations in Materials: This refers to the various ways materials can undergo alterations or modifications.
- Nature of Changes: These transformations may affect different aspects of the substances, including:
- Physical Properties: Changes in color, shape, size, state of matter, and temperature.
- Chemical Properties: Variations in reactivity, composition, and stability.
- Processes Leading to Changes: Various methods or factors can induce these changes:
Heating
- Example 1: Heating ice causes it to melt from solid to liquid.
- Example 2: Heating water transforms it from liquid to gas (steam).
- Impact: Heating induces a change in the state of the substance.
Applying Force
- Example: Compressing a sponge alters its shape.
- Impact: This represents a physical change, affecting the shape and density of the material.
Mixing with Other Substances
- Example: Combining vinegar and baking soda produces carbon dioxide gas and water through a fizzing reaction.
- Impact: This is a chemical change, resulting in the formation of new substances.
Environmental Factors
- Example: Prolonged exposure of iron to moisture and air leads to rusting.
- Impact: This chemical change involves the reaction of iron with oxygen and water, altering its composition and appearance.
Different Types of Changes
Reversible and Irreversible Changes
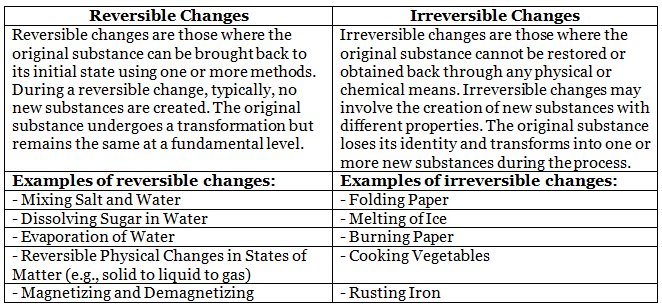
Physical and Chemical Changes

Periodic and Non-periodic Change

Exothermic and Endothermic Change

Change in Size due to Expansion and Contraction
Effects of Heating on a Substance
- Action: Heating increases particle motion and causes them to spread out.
- Result: The substance expands or becomes larger.
Effects of Cooling on a Substance
- Action: Cooling slows down particle movement, bringing them closer.
- Result: The substance contracts or becomes smaller.
Variability in Material Response
- Observation: Different materials exhibit varying degrees of expansion or contraction in response to temperature changes.
Practical Example: Thermometer
- When Heated: As temperature rises, liquid mercury inside a thermometer expands, causing the mercury level to ascend and indicate a higher temperature.
- When Cooled: Conversely, when the temperature drops, the mercury contracts, lowering its level and showing a decreased temperature reading.
Behavior of Liquids
- Similarity to Solids: Like solids, liquids also expand when subjected to heat.
The understanding of how substances expand or contract with temperature variations is crucial for explaining various phenomena, such as the operational mechanism of thermometers and the responses of different materials to heating or cooling.
Change of State on Heating
State Changes in Substances Due to Heating:
- Heating different substances leads to changes in their state (solid, liquid, gas).
Processes and Examples:
Melting:
- Definition: Transition from solid to liquid state upon heating.
- Example: Ice melts into liquid water when heated.
Evaporation:
- Definition: Conversion from liquid to gas state with heat.
- Example: Heating water leads to its evaporation, forming water vapor.
Condensation:
- Definition: Change from gas to liquid state upon cooling.
- Example: Cooling water vapor results in condensation, creating water droplets and leading to cloud or dew formation.
Sublimation:
- Definition: Direct transition from solid to gas state without passing through the liquid phase.
- Example: Dry ice (solid CO2) sublimates to carbon dioxide gas when heated, without becoming liquid.
Understanding these processes is crucial for grasping how heating affects different substances and their transitions between solid, liquid, and gas states.
Anomalous Expansion of Water
- General Behavior of Substances on Cooling: Most substances contract and decrease in volume when cooled.
- Initial Behavior of Water: Like other substances, water contracts upon being cooled from higher temperatures.
- Unique Behavior of Water Below 4°C: At temperatures lower than 4°C, water exhibits unusual behavior by expanding instead of contracting.
- Density of Ice Compared to Water: When water freezes into ice, it becomes less dense than its liquid form, which explains why ice floats on water.
- Scientific Term for Water's Behavior: This peculiar behavior of water is known as the "anomalous expansion of water."
- Ecological Significance of Water's Anomalous Expansion: This property is vital for aquatic life in rivers and lakes. In cold conditions, the top water layer freezes, forming a protective ice layer. This ice shields the water beneath, keeping it relatively warmer, which is crucial for the survival of plants and animals in these ecosystems.
This understanding highlights the exceptional nature of water's expansion upon cooling, especially below 4°C, and its ecological importance.
FAQs on Olympiad Notes: Changes Around Us - Class 6
| 1. What is anomalous expansion of water? |  |
| 2. Why does water exhibit anomalous expansion? |  |
| 3. What are the practical implications of the anomalous expansion of water? |  |
| 4. How does the anomalous expansion of water affect aquatic ecosystems? |  |
| 5. Are there any other substances that exhibit anomalous expansion? |  |



















