Nucleophilic Addition Reactions of Aldehydes and Ketones | Chemistry Optional Notes for UPSC PDF Download
Nucleophilic Addition Reactions of Aldehydes and Ketones
As we saw in the Preview of Carbonyl Chemistry, the most general reaction of aldehydes and ketones is the nucleophilic addition reaction. As shown in Figure 19.2, a nucleophile, :Nu–, approaches the carbonyl group from an angle of about 105° opposite the carbonyl oxygen and forms a bond to the electrophilic C–O carbon atom. At the same time, rehybridization of the carbonyl carbon from sp2 to sp3 occurs, an electron pair from the C═O bond moves toward the electronegative oxygen atom, and a tetrahedral alkoxide ion intermediate is produced. Protonation of the alkoxide by addition of acid then gives an alcohol.
Figure 19.2 MECHANISM A nucleophilic addition reaction to an aldehyde or ketone. The nucleophile approaches the carbonyl group from an angle of approximately 75° to the plane of the sp2 orbitals, the carbonyl carbon rehybridizes from sp2 to sp3, and an alkoxide ion is formed. Protonation by addition of acid then gives an alcohol.
The nucleophile can be either negatively charged (:Nu–) or neutral (:Nu). If it’s neutral, however, it usually carries a hydrogen atom that can subsequently be eliminated, :Nu–H. For example:
Nucleophilic additions to aldehydes and ketones have two general variations, as shown in Figure 19.3. In one variation, the tetrahedral intermediate is protonated by water or acid to give an alcohol as the final product. In the second variation, the carbonyl oxygen atom is protonated and then eliminated as HO– or H2O to give a product with a C═double bond.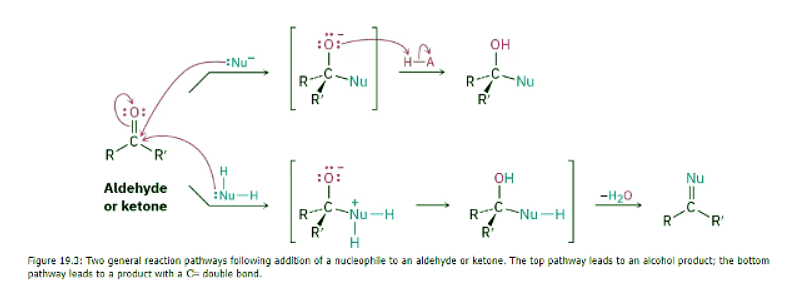
Aldehydes are generally more reactive than ketones in nucleophilic addition reactions for both steric and electronic reasons.

Electronically, aldehydes are more reactive than ketones because of the greater polarization of aldehyde carbonyl groups. To see this polarity difference, recall the stability order of carbocations. A primary carbocation is higher in energy and thus more reactive than a secondary carbocation since it has only one alkyl group inductively stabilizing the positive charge rather than two. In the same way, an aldehyde has only one alkyl group inductively stabilizing the partial positive charge on the carbonyl carbon rather than two, and is a bit more electrophilic, and, therefore, more reactive than a ketone.
One further comparison: aromatic aldehydes, such as benzaldehyde, are less reactive in nucleophilic addition reactions than aliphatic aldehydes because the electron-donating resonance effect of the aromatic ring makes the carbonyl group less electrophilic. Comparing electrostatic potential maps of formaldehyde and benzaldehyde, for example, shows that the carbonyl carbon atom is less positive (less blue) in the aromatic aldehyde.

FAQs on Nucleophilic Addition Reactions of Aldehydes and Ketones - Chemistry Optional Notes for UPSC
| 1. What are nucleophilic addition reactions of aldehydes and ketones? |  |
| 2. How do nucleophilic addition reactions of aldehydes and ketones occur? |  |
| 3. What are some examples of nucleophilic addition reactions of aldehydes and ketones? |  |
| 4. What factors affect the rate of nucleophilic addition reactions of aldehydes and ketones? |  |
| 5. Why are nucleophilic addition reactions of aldehydes and ketones important? |  |

|
Explore Courses for UPSC exam
|

|
















