UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > Sandmeyer, Reimer-Tiemann and Reformatsky reactions
Sandmeyer, Reimer-Tiemann and Reformatsky reactions | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Definition: What is Sandmeyer Reaction? |

|
| Example of Sandmeyer Reaction |

|
| Mechanism of Sandmeyer Reaction |

|
| Applications of Sandmeyer Reaction |

|
Definition: What is Sandmeyer Reaction?
Sandmeyer reaction is a type of radical-nucleophilic aromatic substitution reaction. It is a useful tool by which an amino group on an aromatic ring is replaced with different substituents. During the Sandmeyer reaction, the amino group is converted into a diazonium salt that can be transformed into various functional groups using a catalyst. The mechanism involves several steps, including the generation of intermediary compounds.
Example of Sandmeyer Reaction
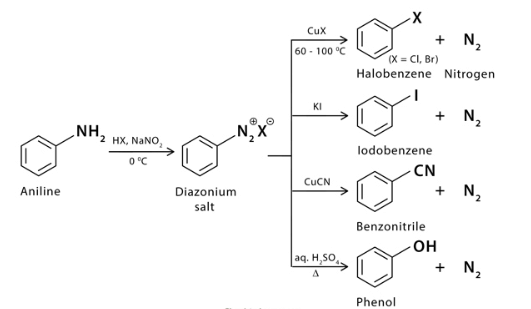
Mechanism of Sandmeyer Reaction
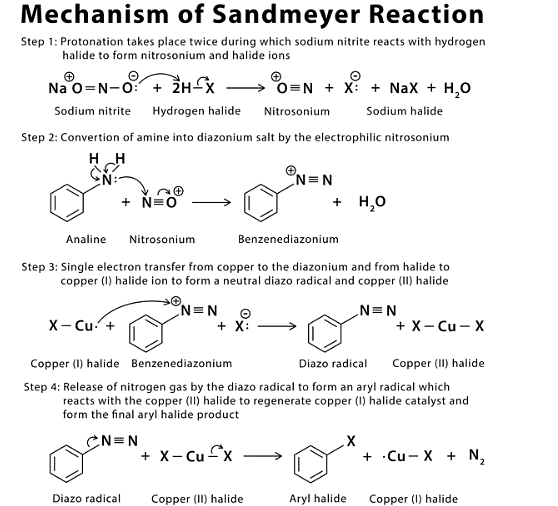
Applications of Sandmeyer Reaction
- Halogenation: Synthesis of aryl halides
- Cyanation: Synthesis of benzonitriles and other useful medicinal drugs
- Trifluoromethylation: Synthesis of aryl compounds having trifluoromethyl group and are used as pharmaceuticals
- Hydroxylation: Conversion of aryl amines into phenols
Question for Sandmeyer, Reimer-Tiemann and Reformatsky reactionsTry yourself: What is the purpose of the Sandmeyer reaction?View Solution
The document Sandmeyer, Reimer-Tiemann and Reformatsky reactions | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on Sandmeyer, Reimer-Tiemann and Reformatsky reactions - Chemistry Optional Notes for UPSC
| 1. What is the Sandmeyer reaction? |  |
The Sandmeyer reaction is a chemical reaction that involves the conversion of an aryl diazonium salt into various functional groups, such as halides, cyanides, and hydroxides. It is an important reaction in organic chemistry for the synthesis of various aromatic compounds.
| 2. Can you provide an example of the Sandmeyer reaction? |  |
Certainly! One example of the Sandmeyer reaction is the conversion of a diazonium salt, such as benzenediazonium chloride, into fluorobenzene. This reaction involves the substitution of a chloride ion with a fluoride ion.
| 3. What is the mechanism of the Sandmeyer reaction? |  |
The Sandmeyer reaction proceeds through a two-step mechanism. In the first step, the diazonium salt is decomposed in the presence of a copper(I) salt to generate a diazonium cation and a copper(II) complex. In the second step, the diazonium cation undergoes a substitution reaction with the desired nucleophile, resulting in the formation of the desired product.
| 4. What are some applications of the Sandmeyer reaction? |  |
The Sandmeyer reaction has several applications in organic synthesis. It is commonly used for the introduction of various functional groups, such as halides, cyanides, and hydroxides, onto aromatic rings. This reaction is also useful for the preparation of pharmaceuticals, dyes, and agrochemicals.
| 5. How does the Sandmeyer reaction differ from the Reimer-Tiemann and Reformatsky reactions? |  |
The Sandmeyer reaction, Reimer-Tiemann reaction, and Reformatsky reaction are all important organic transformations, but they differ in their mechanisms and products. The Sandmeyer reaction involves the substitution of a diazonium salt, while the Reimer-Tiemann reaction involves the generation of a carbonyl compound from a phenol. The Reformatsky reaction, on the other hand, involves the addition of an organozinc halide to an aldehyde or ketone.

|
Explore Courses for UPSC exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches
















