UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > Alkylation and Acylation of Aromatic Rings - The Friedel-Crafts Reaction
Alkylation and Acylation of Aromatic Rings - The Friedel-Crafts Reaction | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Friedel-Crafts Alkylation |

|
| Some limitations of Friedel-Crafts Alkylation |

|
| Friedel-Crafts Acylation |

|
| Solved Example |

|
Friedel-Crafts Alkylation
- Friedel-Crafts Alkylation was first discovered by French scientist Charles Friedel and his partner, American scientist James Crafts, in 1877. This reaction allowed for the formation of alkyl benzenes from alkyl halides, but was plagued with unwanted supplemental activity that reduced its effectiveness.

The mechanism takes place as follows:
Step 1:

- Step one creates a carbocation that acts as the electrophile in the reaction. This steps activates the haloalkane. Secondary and tertiary halides only form the free carbocation in this step.
Steps 2 and 3:

- Step 2 has an electron pair from the aromatic ring attack the carbocation forming a new C-C bond. The arenium ion intermediate results with stabilization from multiple resonance forms. The loss of a proton then gives the neutral alkylated substitution product.
Final Products

- The reactivity of haloalkanes increases as you move up the periodic table and increase polarity. This means that an RF haloalkane is most reactive followed by RCl then RBr and finally RI. This means that the Lewis acids used as catalysts in Friedel-Crafts Alkylation reactions tend have similar halogen combinations such as BF3, SbCl5, AlCl3, SbCl5, and AlBr3, all of which are commonly used in these reactions.
Question for Alkylation and Acylation of Aromatic Rings - The Friedel-Crafts ReactionTry yourself: What is the purpose of Friedel-Crafts Alkylation reaction?View Solution
Some limitations of Friedel-Crafts Alkylation
- There are possibilities of carbocation rearrangements when you are trying to add a carbon chain greater than two carbons. The rearrangements occur due to hydride shifts and methyl shifts. For example, the product of a Friedel-Crafts Alkylation will show an iso rearrangement when adding a three carbon chain as a substituent. One way to resolve these problems is through Friedel-Crafts Acylation.
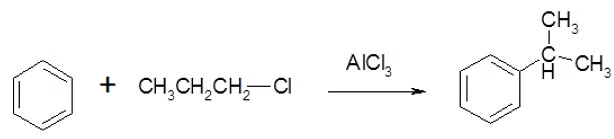
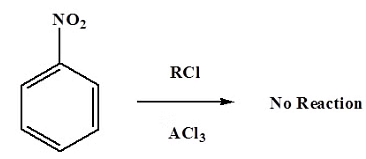
- Also, the reaction will only work if the ring you are adding a substituent to is not deactivated. Friedel-Crafts fails when used with compounds such as nitrobenzene and other strong deactivating systems.
- Friedel-Crafts reactions cannot be preformed then the aromatic ring contains a NH2, NHR, or NR2 substituent. The lone pair electrons on the amines react with the Lewis acid AlCl3. This places a positive charge next to the benzene ring, which is so strongly activating that the Friedel-Crafts reaction cannot occur.
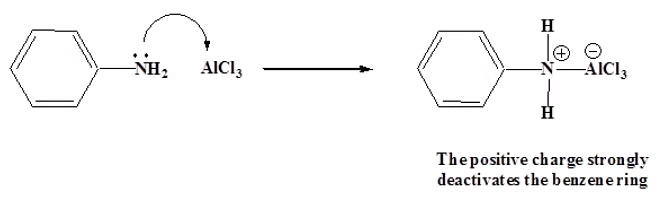
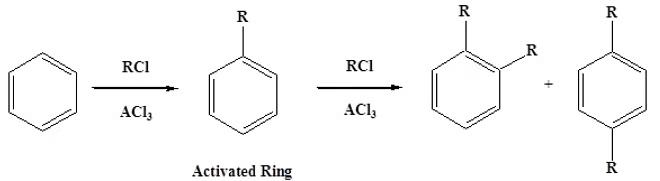
- Lastly, Friedel-Crafts alkylation can undergo polyalkylation. The reaction adds an electron donating alkyl group, which activates the benzene ring to further alkylation.

- This problem does not occur during Friedel-Crafts Acylation because an acyl group is deactivating, thus prevents further acylations.

Question for Alkylation and Acylation of Aromatic Rings - The Friedel-Crafts ReactionTry yourself: What is a limitation of Friedel-Crafts Alkylation?View Solution
Friedel-Crafts Acylation
- The goal of the reaction is the following:

- The very first step involves the formation of the acylium ion which will later react with benzene:
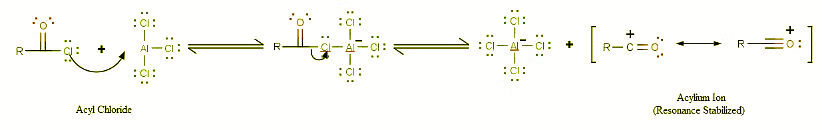
- The second step involves the attack of the acylium ion on benzene as a new electrophile to form one complex:
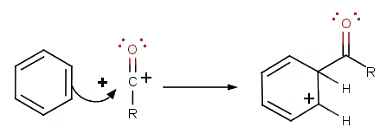
- The third step involves the departure of the proton to reform aromaticity:
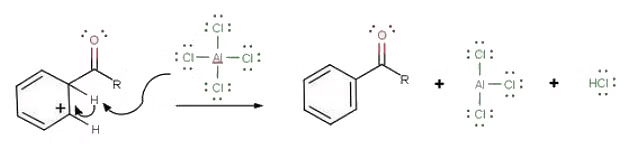
- During the third step, AlCl4 returns to remove a proton from the benzene ring, which enables the ring to return to aromaticity. In doing so, the original AlCl3 is regenerated for use again, along with HCl. Most importantly, we have the first part of the final product of the reaction, which is a ketone. The first part of the product is the complex with aluminum chloride as shown:

- The final step involves the addition of water to liberate the final product as the acylbenzene:

- Because the acylium ion (as was shown in step one) is stabilized by resonance, no rearrangement occurs (unlike in Friedel-Crafts Alkylation reactions - see Limitation 1 above). Also, because of of the deactivation of the product, it is no longer susceptible to electrophilic attack and hence, no longer goes into further reactions (Limitation 3 above from Friedel-Crafts Alkylation reactions). However, as not all is perfect, Limitation 2 still prevails where Friedel-Crafts Acylation fails with strong deactivating rings.
Solved Example
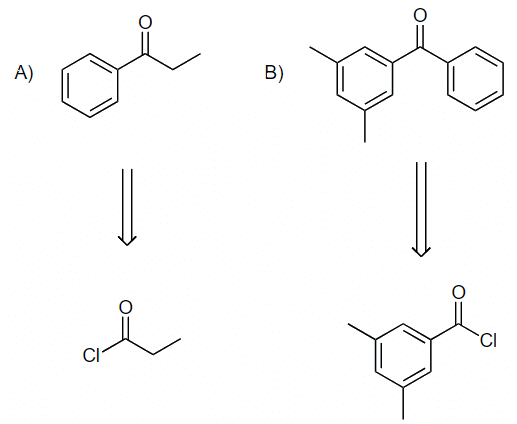
Example: Suggest an acyl chloride that was used to make the following compounds:
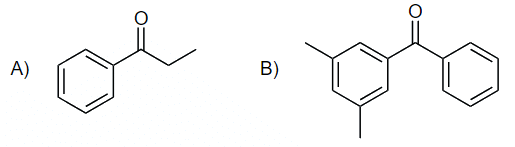 Ans:
Ans:
The document Alkylation and Acylation of Aromatic Rings - The Friedel-Crafts Reaction | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on Alkylation and Acylation of Aromatic Rings - The Friedel-Crafts Reaction - Chemistry Optional Notes for UPSC
| 1. What is Friedel-Crafts Alkylation? |  |
Ans. Friedel-Crafts alkylation is a chemical reaction that involves the introduction of an alkyl group onto an aromatic ring. It is named after the chemists Charles Friedel and James Crafts, who discovered this reaction in the 19th century. This reaction is commonly used in organic synthesis to create new carbon-carbon bonds and modify aromatic compounds.
| 2. What are some limitations of Friedel-Crafts Alkylation? |  |
Ans. Some limitations of Friedel-Crafts alkylation include:
- The reaction is limited to aromatic compounds and does not work well with other types of substrates.
- It is highly sensitive to the presence of electron-withdrawing groups on the aromatic ring, which can lead to side reactions or low yields.
- Friedel-Crafts alkylation can also lead to over-alkylation, where multiple alkyl groups are added to the aromatic ring, resulting in a mixture of products.
- The reaction requires the use of strong Lewis acids, such as aluminum chloride, which can be corrosive and difficult to handle.
- Steric hindrance can also be a limitation, as bulky alkyl groups may have difficulty accessing the aromatic ring, leading to low reactivity.
| 3. What is Friedel-Crafts Acylation? |  |
Ans. Friedel-Crafts acylation is a chemical reaction similar to alkylation, but instead of introducing an alkyl group, it introduces an acyl group onto an aromatic ring. This reaction is also named after Charles Friedel and James Crafts and is widely used in organic synthesis to create ketones and other acylated products.
| 4. What are the differences between Friedel-Crafts alkylation and acylation? |  |
Ans. The main differences between Friedel-Crafts alkylation and acylation are:
- In alkylation, an alkyl group is added to the aromatic ring, while in acylation, an acyl group (such as a carbonyl group) is added.
- Friedel-Crafts alkylation requires the use of a strong Lewis acid catalyst, such as aluminum chloride, while acylation can be catalyzed by milder Lewis acids or even Lewis acid-free conditions.
- Alkylation can lead to the over-alkylation of the aromatic ring, resulting in a mixture of products, while acylation usually gives a single acylated product.
- Alkylation is more sensitive to steric hindrance compared to acylation, as bulky alkyl groups may have difficulty accessing the aromatic ring.
| 5. What are some applications of Friedel-Crafts reactions? |  |
Ans. Friedel-Crafts reactions, including alkylation and acylation, have several applications in organic synthesis. Some of these include:
- Introduction of new functional groups onto aromatic rings to create pharmaceuticals, dyes, and other fine chemicals.
- Preparation of aromatic ketones and other acylated products, which are important intermediates in various industrial processes.
- Synthesis of natural products and complex organic compounds with aromatic rings.
- Modification of polymers and materials by introducing aromatic groups onto their structures.
- Development of new catalysts and reagents for organic transformations.

|
Explore Courses for UPSC exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches