E1cb Reaction and mechanism | Chemistry Optional Notes for UPSC PDF Download
Introduction
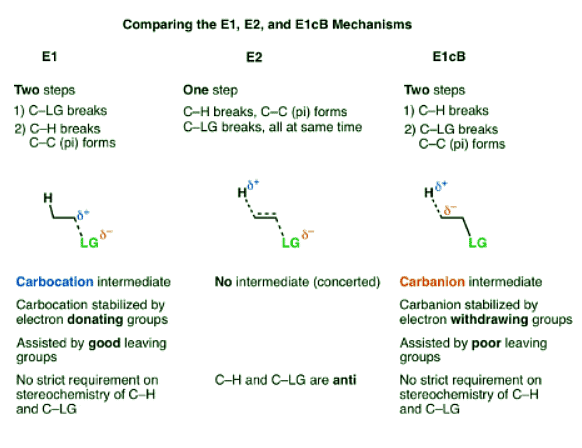
- The E1cB (Elimination, Unimolecular, Conjugate Base) mechanism is a third mechanistic pathway for elimination reactions.
- In many ways it is the exact opposite of the E1 mechanism, as the first step is deprotonation to form a carbanion, followed by elimination in the second step.
- It does occasionally come up in introductory organic chemistry courses, particularly in the mechanism of the aldol condensation, aryne formation, and elimination of alkenyl halides to give alkynes.

Elimination To Give Alkenes: The E1 and E2 Pathways
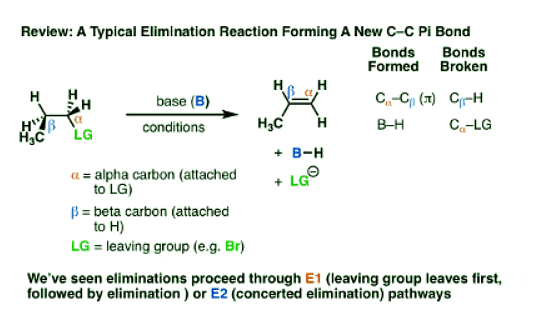
- Elimination reactions are one of the most common and powerful methods we’ve learned to make alkenes (carbon-carbon pi bonds) from alkyl halides.
- In elimination reactions giving new C-C pi bonds, the same general pattern of bond-forming and bond-breaking is always observed!
- A single bond between the carbon and a leaving group breaks (C-LG). The carbon attached to the leaving group (LG) is referred to as the “alpha“(α) carbon.
- A single bond between the carbon and a hydrogen breaks. The carbon bearing this hydrogen is referred to as the “beta” (β)carbon.
- A new pi bond forms between the alpha carbon and the beta carbon.

- Everyone agrees on what happens. The key question we’d really like to know, however, is “how did it happen?”.
- [Screenwriters know this. They give away the endings to movies all the time – often in the first 5 minutes. But what keeps you watching is trying to understand how something happened. Why else would people sit through a six hour movie trilogy if they already know Anakin is going to become Darth Vader in the end?]
- The sequence in which these bonds form and break is the “mechanism” of a reaction, or if you will, the “story” of how this reaction happens.
- We’ve seen two of these patterns so far for elimination:
- In the E1 mechanism, elimination happens in two steps. The C-LG bond breaks first, leaving behind a carbocation. This is the slow step. In the second step, a base deprotonates the beta carbon, breaking C-H. The lone pair of electrons from the C-H bond forms the new pi bond with the empty p orbital from the carbocation. The rate law is unimolecular (hence E1) since it depends only on the substrate.
- In the E2 mechanism, the C-H bond and C-LG bond break at the same time that the new C-C pi bond is formed. This is a concerted mechanism. The rate law is bimolecular (hence E2) since the reaction rate depends both on the concentration of base and of substrate.
The E1cB Mechanism
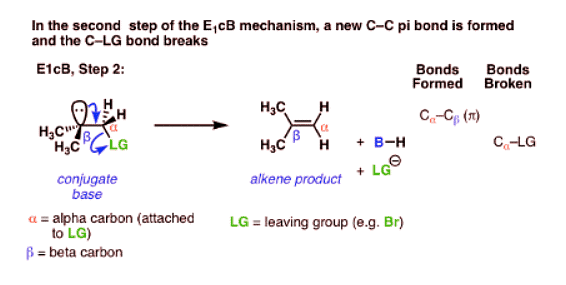
- The third possibility is that the beta carbon is deprotonated first (form B–H, break C–H). In the limiting case, this would give an anionic intermediate, the “conjugate base” of the substrate.

- Since the first step is deprotonation to give an anion, then you would be right to guess that this mechanism is more likely to occur when the C–H bond is relatively acidic, as when the alpha carbon is adjacent to an electron withdrawing group like a ketone, nitrile (CN), or nitro group (NO2). These groups stabilize negative charge.
- In the second step, the pair of electrons from the conjugate base then displaces the leaving group, forming a pi bond [form C–C pi, break C–LG].

- In the (many) cases of the E1cB where the second step is the slow step, you can imagine that this step would be made slower by a relatively poor leaving group like (HO-), (CH3O-) or even (believe it or not!) fluorine [Note 1].
The E1cB Mechanism And The E1 Mechanism Are Two Extremes, With The E2 Mechanism Lying In The Middle
- It’s helpful to think of the E1cB mechanism and the E1 mechanisms as two extremes, or mirror images, and the E2 cutting exactly in between. It’s kind of like the continuum between “quitting” and “being fired”, with “mutually agreeing to part ways” somewhere in the middle.
- The first step in the E1 mechanism is loss of a leaving group (break C-LG) to give a carbocation intermediate, and this step is promoted when there are electron-donating groups adjacent to the alpha carbon (making the carbocation more stable).
- The first step in the E1cB mechanism is deprotonation (break C-H) to give a carbanion intermediate, and this step is promoted when there are electron-withdrawing groups adjacent to the alpha carbon (making the carbanion more stable).
- The E2 mechanism is in between these two extremes, where everything happens at once.

Examples Of The E1cB Mechanism In Introductory Organic Chemistry: The Aldol Condensation
- Probably the most commonly encountered example of the E1cB mechanism in introductory organic chemistry is in the aldol condensation reaction, specifically the last step where the new C-C double bond is formed.
- Below, left, is the product of an alcohol addition reaction. If this is heated with base (like NaOCH3), it loses water, giving the alkene. (Loss of water = “condensation”)

- Wait a minute, you may say. “I thought the hydroxyl ion (HO-) was a poor leaving group? What’s going on here?”
- Exactly! If there was a really good leaving group (like OTs or Br) present instead of OH, an E2 (or even E1) mechanism would be more likely. [Note 2] The bad leaving group (HO-) makes the elimination step is slow, which is largely responsible for this being a two-step mechanism.
- Another (very subtle) point is stereochemistry. If you look really closely you’ll notice in the drawing above that the C–H bond and the C–OH bond are not anti to each other (a requirement for the E2) but instead are syn.
- So unlike the E2, the E1cB mechanism doesn’t have a strict requirement that the C-H and C-LG bonds be anti to each other.
Another Example: Formation Of Alkynes From Alkenyl Halides
- Another example of the E1cB is in the formation of alkynes from alkenyl halides with NaNH2 in liquid ammonia (NH3).
- When alkenyl halides are treated with NaNH2 in NH3 as solvent, elimination to give the alkyne occurs. This reaction works even if the H and Cl are cis to each other, which would seem to rule out the E2 mechanism. [The elimination when H and the halide are trans is much faster, though! [Note 3]
- NaNH2 is an extremely strong base. Being sp2 hybridized, the C-H bond of the alkenyl halide is reasonably acidic (pKa about 40) and able to stabilize negative charge.
- In this case a two-step mechanism likely operates, where 1) a carbanion intermediate is formed by deprotonation, followed by 2) loss of the leaving group.

Formation Of Arynes From Phenyl Halides
- A related situation to alkenyl halides where the E1cB mechanism can arise is in the formation of benzyne and other “arynes”. Although benzyne does not have a “true” pi bond, many of the same principles of the E1cB still apply.
- Deprotonation of the aryl fluoride by the strong base (NaNH2) occurs first, giving a carbanion. In this specific case, the second step is loss of the bad leaving group, fluorine. [Note 1]

Note: Apparently, when F or Cl is the leaving group, deprotonation is the slow step. However, when the leaving group is Br, or I, deprotonation is rate-determining and breakage of C-X is fast. Note that both of these still qualify as E1cB so long as the reaction proceeds through the carbanion intermediate!
The Reaction Coordinate Diagram Of The E1cB Reaction
So what does the reaction coordinate diagram of the E1cB look like? If we assume that the first step is fast and the second step is slow (rate-determining), and there is an anion intermediate, then the reaction coordinate diagram of the E1cB should look roughly like this: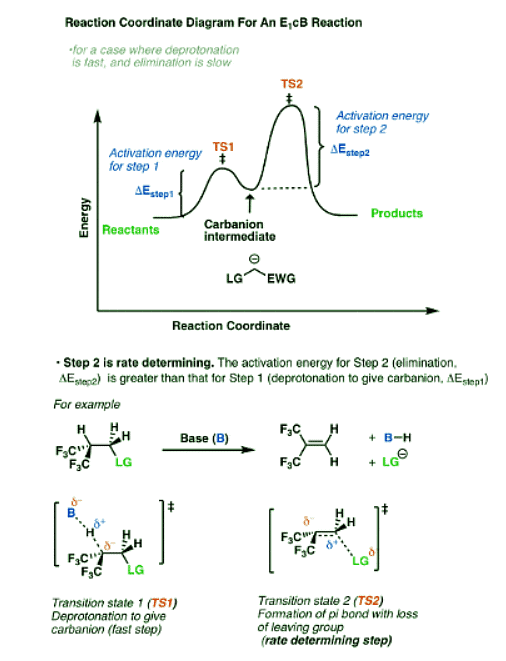
- We’ve drawn a small hump going from reactants to TS1 with activation energy (ΔEStep1 ) equal to [Δ E TS1 – Δ E reactants].
- This then proceeds to the anionic intermediate (the “well” in the middle)
- Which in turn proceeds through TS2 with activation energy (Δ EStep2 ) equal to [Δ E TS2 – Δ E intermediate]
- Since ΔEStep2 > ΔEStep1, the second step is rate determining
Compare that to the reaction coordinate of the E1 elimination, which is the mirror image.
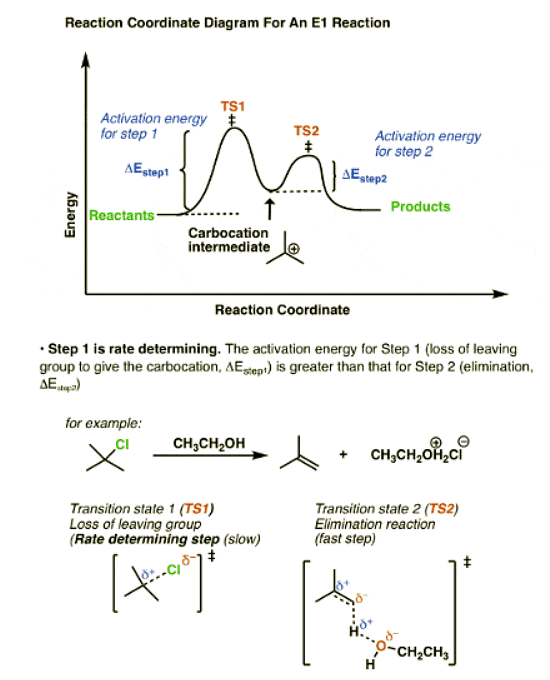
In the E1 mechanism, the energy barrier (activation energy, ΔEStep1 ) for the first step (loss of leaving group) is higher than the energy barrier (ΔEStep2) for the second step (elimination).
The reaction coordinate in the E2 case shows both of these steps happening at the same time.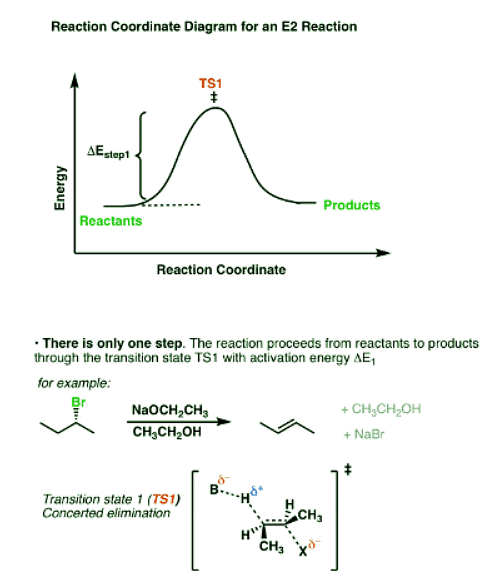
Notes
- Note 1: We never consider F(–) as a leaving group for SN2 reactions, but under certain conditions it can be a leaving group in various types of substitution and elimination reactions. One example is in nucleophilic aromatic substitution, another example of a mechanism that proceeds through a relatively stable anion.
- Note 2: Which suggests that in eliminations with a good leaving group, E2 is followed even when a strong electron withdrawing group is present.
- Note 3: The reaction is much easier when H and Br are trans to each other (can be done with NaOH!) but requires NaNH2/NH3 when the Br and H are cis.
Summary: When Is a Reaction Likely To Go Through An E1cB Mechanism?
So, to summarize, in the E1cB mechanism, elimination occurs in two steps:
- Deprotonation of the substrate to give an anion (its conjugate base), followed by
- Loss of a leaving group to give a new C-C pi bond.
The E1cB mechanism is not very commonly encountered in introductory organic chemistry, but when it is observed, expect to see several of these characteristics:
- The presence of an electron-withdrawing group which helps to stabilize an intermediate anion
- A relatively poor leaving group (which will slow down the second step)
Here’s one last classic example of an E1cB, where deprotonation leads to a very stable anion (it’s aromatic) which slowly loses the poor leaving group (hydroxide ion) forming a new C-C pi bond.
FAQs on E1cb Reaction and mechanism - Chemistry Optional Notes for UPSC
| 1. What is the E1cB mechanism? |  |
| 2. How does the E1cB mechanism differ from the E1 mechanism? |  |
| 3. What is the role of the E2 mechanism in the E1cB mechanism? |  |
| 4. Can you provide an example of the E1cB mechanism in introductory organic chemistry? |  |
| 5. How is the E1cB reaction likely to occur? |  |

|
Explore Courses for UPSC exam
|

|