What is Optical Isomerism?
Optical isomerism is a case where the isomers display identical characteristics in terms of molecular weight as well as chemical and physical properties. However, they differ in their effect on the rotation of polarized light.
- Optical isomerism occurs mainly in substances that have the same molecular and structural formula, but they cannot be superimposed on each other.
- In simple words, we can say that they are mirror images of each other. Alternatively, it can also be found in substances that have an asymmetric carbon atom.
 Depiction of Mirror Images of Alanine.
Depiction of Mirror Images of Alanine.
What is Plane Polarized Light?
If by filtering the beam with specialized materials, the electric field vectors are limited to a single plane, then the light is referred to as plane or linearly polarised with respect to the propagation direction. All waves vibrating in a single plane are referred to as plane-parallel or plane-polarized.
 Plane Polarized Light
Plane Polarized Light
- Optical isomerism is shown by stereoisomers which rotate the plane of polarized light.
- If the plane of polarized light passing through the enantiomer solution rotates in the clockwise direction then the enantiomer is said to exist as (+) form and if the plane of polarized light rotates in an anti-clockwise direction then the enantiomer is said to exist in (-).

- If the rotation of light is anti-clockwise then laevo rotatory (l-form)
- If the rotation of light is clockwise then the dextro rotatory substance(d-form)
- If there is no rotation of light then the substance is called optically inactive.
Chiral and Achiral Molecules
Chiral and achiral molecules are used to describe the symmetry characteristics of molecules. There are two main criteria to determine if a molecule is chiral or achiral:
- On the Basis of Atoms Attached
- On the Basis of Plane of Symmetry
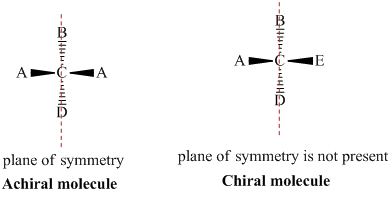
- A chiral molecule is a molecule that is not superimposable on its mirror image. In other words, it does not possess an internal plane of symmetry.
- Achiral molecules are those that possess a plane of symmetry and are superimposable on their mirror image.
 Superimposable and Nonsuperimposable Nature
Superimposable and Nonsuperimposable Nature
Chiral and achiral molecules can be identified based on two factors:
1. Based on Atoms Attached:
- If in a molecule, all the four valencies of carbon are satisfied by four different atoms or four different group atoms then carbon is known as chiral carbon and the molecule is called Chiral.
 Achiral and Chiral Compounds
Achiral and Chiral Compounds
- If in a molecule, all the four valencies of carbon are not satisfied by four different atoms or four different group atoms then carbon is known as achiral carbon and molecule is called Achiral.
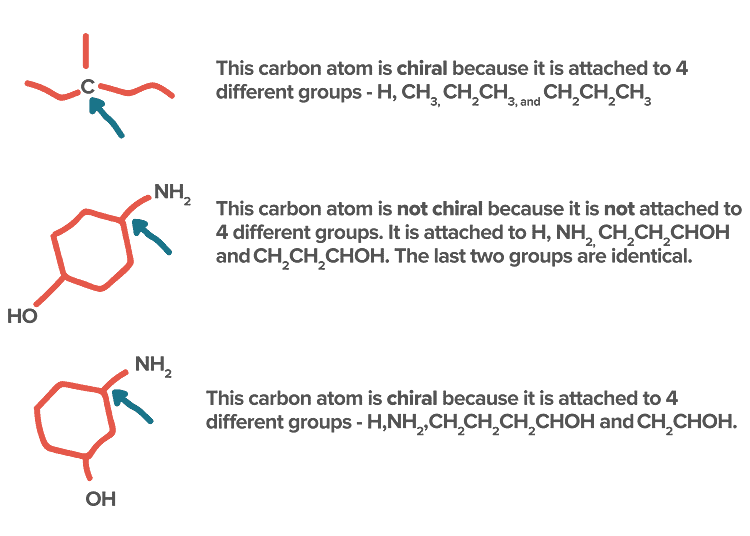 Identification of Chiral and Achiral CarbonsSome other Examples:
Identification of Chiral and Achiral CarbonsSome other Examples:
 2 Chiral Carbon
2 Chiral Carbon 2 Chiral Carbon
2 Chiral Carbon  No Chiral Carbon
No Chiral Carbon
2. Based on Plane of Symmetry:
- If the molecule has a chiral center (four different groups are attached) and does not have a plane of symmetry or axis of symmetry then it is called a Chiral molecule.
- If the molecule has a plane of symmetry or axis of symmetry then it is called achiral molecule. Achiral molecules do not have a chiral center.
 Chiral and Achiral Molecule
Chiral and Achiral Molecule
Examples:
 Achiral due to Plane of Symmetry
Achiral due to Plane of Symmetry
What are Enantiomers and Diastereomers?
Enantiomers and diastereomers are terms used to describe different types of stereoisomers, which are molecules that have the same molecular formula and connectivity but differ in their spatial arrangement.
 Flow Chart Explaining Enantiomers and Diastereomers
Flow Chart Explaining Enantiomers and Diastereomers
Enantiomers
Enantiomers are a pair of molecules that exist in two forms that can not be superimposed on each other but are mirror images of each other.
 Enantiomers: Non-Superimposable Mirror Images
Enantiomers: Non-Superimposable Mirror Images
- Enantiomers are molecules with the same chemical formula and connectivity but differ in their three-dimensional arrangement. They cannot be superimposed on each other, similar to how our hands are mirror images but cannot be perfectly overlapped.
- Enantiomers share similar physical and chemical properties, but they exhibit different optical behavior. When plane-polarized light is passed through enantiomers, they demonstrate different optical properties, such as the direction and degree of rotation of the light.
- If we imagine placing a mirror between the two enantiomers and looking at one of them, we would see the other enantiomer reflected in the mirror. This is because the original enantiomer and its mirror image have distinct spatial arrangements and are considered enantiomers.
- It is important to note that if an object and its mirror image can be superimposed, they are not enantiomers. Enantiomers are specifically defined as non-superimposable mirror images.
However, if an object and its mirror image cannot be superimposed, they are optically active and referred to as enantiomers.
To determine if an object and its mirror image are superimposable, we can perform a test. Either the object or its mirror image is rotated by 180 degrees along with the mirror. After this rotation, we examine if the mirror image can be overlapped perfectly with the original object. Let us consider two examples:
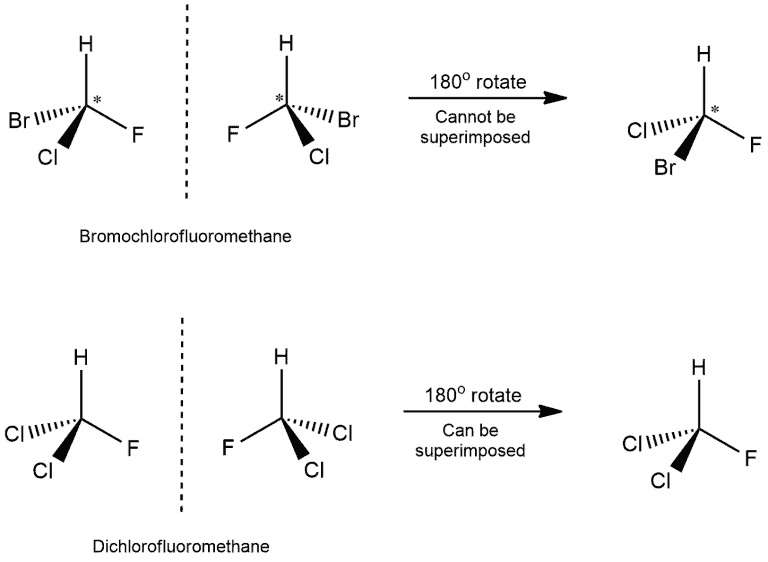 Identifying Superimposable Images
Identifying Superimposable Images
- In the first example, we can see that four different atoms are attached to the carbon atom. Those are bromine, chlorine, fluorine, and hydrogen. The chiral center is marked by a star (*), which is the usual way of marking it.
- When we would put the mirror next to the molecule on the left, we would get the molecule on the right. Then, if we would rotate this molecule by 180 degrees, we wouldn’t get the same molecule as on the left. Hydrogen and fluorine will be in the same position but chlorine and bromine on the opposite. And because of that, we get non-superimposable structures.
- In the other example, we have two chlorine atoms attached to the carbon. When we rotate the mirror image, we get the same molecule as on the left! This means that this molecule has a superimposable structure.
Diastereomers
A diastereomer is a stereoisomer with two or more stereocenters and the isomers are not mirror images of each other.
- If a molecule has more than one stereocenter and every single stereocenter isn’t in the opposite direction then they are not enantiomers, but diastereomers.
- In the following example, we have three stereocenters. On the first and third, the configuration is different (which would resemble the enantiomers) but on the second stereocenter is the same configuration. This means that they aren’t enantiomers but diastereomers.
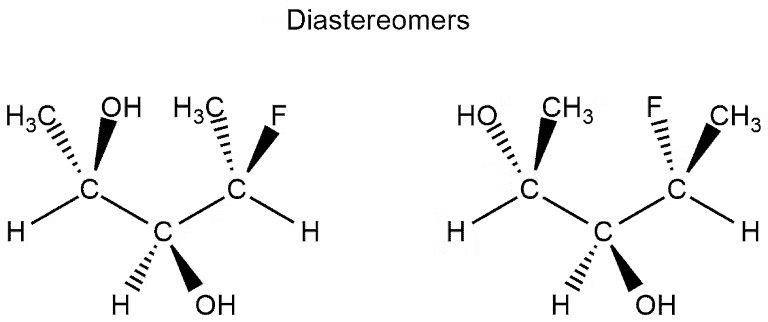 Example of Diastereomers
Example of Diastereomers
Example: Enantiomers and Diastereomers through Mirror Images:
 Identifying Enantiomers and Diastereomers through Mirror Images
Identifying Enantiomers and Diastereomers through Mirror Images
Some Solved Examples of Determining Enantiomers
Q.1. Which of the following pairs of compounds is a pair of enantiomers?
A.
B.
C. 
D.

Solution: The correct option is B
- In option (d), one of the isomers has a plane of symmetry so it won't show chirality and hence, it cannot be its enantiomeric pair.
- Cyclohexane molecules exist in chair form and they do not have a plane of symmetry in the ring. Both the isomers in option (c) are not mirror images. Hence, they are not enantiomers.
- In option (a), both the structures are identical. They have the same configuration as the chiral centers.
 Identical Structures
Identical Structures - In (b), both are mirror images along the plane of paper and are not superimposable on each other. So, they are enantiomers.
Hence, (b) is correct.
Q.2. Which of the following is capable of existing as a pair of enantiomers?
A. 3-Methylpentane
B. 3-Methylhexane
C. 2-Methylpentane
D. 2-Methylpropane
Solution: 3- Methylhexane is optically active because of molecular asymmetry and chiral carbon. Hence it is capable of existing as a pair of enantiomers. 3-Methylhexane
3-Methylhexane
Determining Enantiomers and Diastereomers through R, S Assignments
The enantiomer of a molecule will always have an opposite R/S configuration. So to get the enantiomer all we need to do is flip all the stereocenters.
 R-S Configuration of Enantiomers
R-S Configuration of Enantiomers
Diastereomers arise when at least two molecules share at least one (but not all) chiral center(s) with identical (R/S) configuration. So to find the diastereomers of (2R,3R,4R)-2,3,4,5-tetrahydroxypentanal, all we need to do is keep at least one stereocenter the same and flip any or all of the rest.
 Determining Enantiomers and Diastereomers through R-S ConfigurationThe following rule can be applied to all molecules with two stereocenters:
Determining Enantiomers and Diastereomers through R-S ConfigurationThe following rule can be applied to all molecules with two stereocenters:
R-S configuration in Enantiomers and Diastereomers

The R and S designations, derived from the Cahn-Ingold-Prelog priority rules, are commonly used to differentiate enantiomers and establish their spatial configurations.
The Cahn Ingold Prelog (CIP) System For Naming Chiral Centers
Priority order:
- The Cahn Ingold Prelog (CIP) system is used to name chiral centers based on their priority.
- The groups around the chiral center are ranked from highest to lowest atomic number.
- We look at their atomic numbers, with the highest being priority 1 and the lowest being priority 4. If two groups have the same atomic number, we check the next atom's atomic number to determine priority.
- Few examples for assigning Priority Order:





To determine the configuration (R/S) of the chiral center:
- Position the chiral center so that the #4 priority substituent points away from you.
- Trace the path from priorities #1 to #2 to #3, ignoring #4.
- If the path traced is clockwise, the chiral center is assigned (R). If it is counterclockwise, the chiral center is assigned (S).
Racemic Mixture & Meso Compounds
There are organic compounds that have similar chemical formulas but different molecular structures. They are called enantiomers. When enantiomers are present in equal quantities in a mixture, it is called a racemic mixture.
- It is an equimolar mixture of R and S or d and l.
- A racemic mixture is optically inactive.
A compound is optically active due to:
(1) Absence of plane of symmetry (POS)
(2) Absence of centre of symmetry (COS)
- POS is an imaginary plane where if we place a mirror, the mirror image will exactly overlap the other half.
- For meso form, there must be at least two identical chiral carbons.
Identical carbon ⇒ Chiral carbons having identical groups attached. - If the compound has POS then it will be certainly optically inactive and will be called meso form.
 Meso Compound (POS Present)
Meso Compound (POS Present)
- After finding two identical carbons. We assign them as R or S. If the first part is R and the other is S then they will rotate the light in the opposite direction but to an equal extent the compound will be optically inactive. (meso form)

Allenes, Biphenyls, and Spiro Compounds
Allenes, biphenyls, and spiro compounds are interesting classes of organic compounds with unique structural features. Let's explore each of them:
1. Allene system


They are non-superimposable mirror image


Optically active
 Inactive
Inactive
2. Spiro Compounds


If no. of rings are even ⇒ optically active
If no. of rings are odd ⇒ Inactive


 Optically Active⇒ This is the even no. of double bonds case.
Optically Active⇒ This is the even no. of double bonds case.
For optical activity, the carbons at extreme positions must have different groups attached.
► 
⇒ Planar compound
Always have POS, Optically inactive.
3. Biphenyls

If biphenyl contains a bulky group at its ortho position (only) then due to repulsion the planarity of the compound disappears and its mirror image is non-superimposable.
► In the biphenyls none of the two rings must have symmetry.

Optically inactive
► 
Optically active
In 2º Amines.

Optically inactive due to the formation of a racemic mixture.
► Order of flipping in amines: 1º > 2º > 3º
FAQs on What are Optical Isomers? - JEE
| 1. What is optical isomerism? |  |
| 2. What is plane polarized light? |  |
| 3. What are chiral and achiral molecules? |  |
| 4. What are enantiomers and diastereomers? |  |
| 5. How can enantiomers and diastereomers be determined through R, S assignments? |  |
| 6. What is a racemic mixture and meso compound? |  |

|
Explore Courses for JEE exam
|

|