Carbonyl Groups: Nomenclature & Preparation | Chemistry for JEE Main & Advanced PDF Download
Carbonyl Compounds
A carbonyl group is a special arrangement of atoms in a molecule, where a carbon atom is tightly connected to an oxygen atom through a double bond. This pairing makes the carbon atom share two electrons with the oxygen atom.
The general formula of carbonyl compounds is CnH2nO.
 Representation of Carbonyl Group
Representation of Carbonyl Group
Carbonyl compounds can be broadly categorized into two main groups:
1. Aldehydes: Carbonyl compounds where the carbonyl group is bonded to at least one hydrogen atom.
2. Ketones: Carbonyl compounds where the carbonyl group is bonded between two carbon atoms.
These categories are based on the arrangement of atoms around the carbonyl functional group, influencing their chemical properties and reactions.
 Aldehydes and Ketones
Aldehydes and Ketones
Nomenclature of Carbonyl Groups
Common Names
Aldehydes and ketones are often known by simpler names instead of their complex IUPAC names. In the case of aldehydes, common names are derived from corresponding carboxylic acids by replacing "–ic" with "aldehyde." These names also reflect the origin using Latin or Greek terms. Substituent positions in the carbon chain are indicated by Greek letters like α, β, γ, δ, etc., with the "α-carbon" directly linked to the aldehyde group.
 Common Names of Aldehydes
Common Names of Aldehydes
The common names of ketones come from naming two alkyl or aryl groups connected to the carbonyl group. Greek letters like α, α', β, β', etc., indicate the positions of substituents, starting with the carbon atoms next to the carbonyl group (labeled as αα'). Some ketones have historical names; for instance, the simplest dimethyl ketone is called acetone. When dealing with alkyl phenyl ketones, they are typically named by adding the acyl group name as a prefix to the term "phenone."
 Common Names of Ketones
Common Names of Ketones
IUPAC Names
The names of aliphatic aldehydes and ketones, according to IUPAC, are derived from the corresponding alkanes by changing the ending -e to -al for aldehydes and -one for ketones.
- Aliphatic aldehydes end with -al, and ketones end with -one.
- Aldehyde chain numbering starts from the aldehyde carbon; ketone numbering starts from the end near the carbonyl group.
- Substituents are listed alphabetically with positional numbers.
- Cyclic ketones have the carbonyl carbon as number one.
- For aldehydes in a ring, use the term carbaldehyde.
- Aromatic aldehydes on benzene are named benzenecarbaldehyde, commonly known as benzaldehyde.
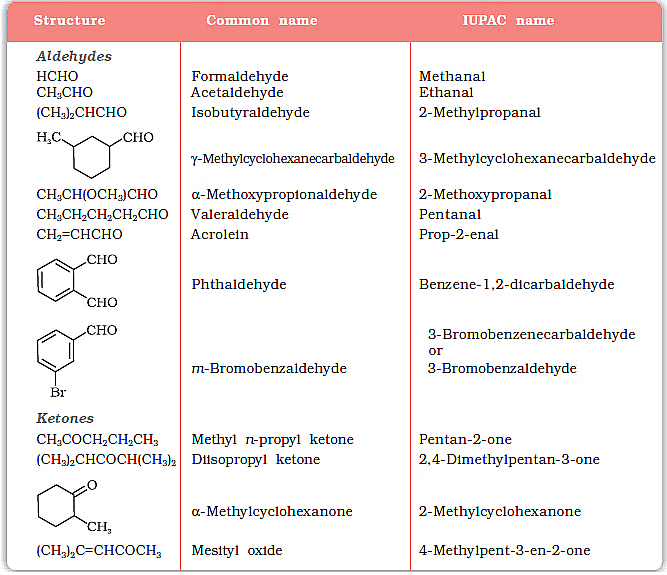 Examples of Aldehyde and Ketones
Examples of Aldehyde and Ketones
Structure of Carbonyl Groups
The carbon in the carbonyl group has a unique setup called sp2 hybridization, forming three strong sigma (σ) bonds. Meanwhile, its fourth electron hangs out in a p-orbital, teaming up with oxygen's p-orbital to make a π-bond.
Oxygen brings two unshared pairs of electrons to the mix. This makes the carbonyl carbon and its three buddies all sit in a flat plane.
The π-electron cloud spreads both above and below this plane. We call this arrangement a trigonal coplanar structure, and the bond angles are pretty close to 120°.
 Orbital Diagram for the Formation of the Carbonyl Group
Orbital Diagram for the Formation of the Carbonyl Group
Polarization occurs in the carbon-oxygen bond because oxygen is more electronegative than carbon. This makes the carbonyl carbon act like a Lewis acid, being electrophilic. Meanwhile, the carbonyl oxygen acts like a Lewis base, also having electrophilic properties.
Carbonyl compounds are more polar than ethers, thanks to their strong dipole moments. This heightened polarity arises from the resonance between neutral and dipolar structures.

Preparation of Both Aldehydes and Ketones
Hydrolysis of Gem Dihalides
- The hydrolysis of geminal dihalides involves the reaction where the dihalide compound reacts with water to produce aldehydes and ketones.
- In this process, one of the halide groups is replaced by a hydroxyl group (OH), resulting in the formation of carbonyl compounds. This reaction is significant in organic chemistry as it provides a method for synthesizing aldehydes and ketones from geminal dihalides.
- The hydrolysis of gem dihalides is often catalyzed by acids or bases, facilitating the cleavage of the carbon-halogen bond and subsequent formation of the carbonyl functional group.
Oxidation of Diols
The oxidation of diols, compounds with two hydroxyl groups, leads to the formation of aldehydes and ketones. This process involves the removal of hydrogen atoms from the hydroxyl groups, resulting in the creation of carbonyl groups.
Common oxidizing agents, such as metal oxides or periodic acids, are used to catalyze this transformation.
 Oxidation of Diols
Oxidation of Diols
Ozonolysis of Alkenes
- Ozonolysis of alkenes is a chemical reaction that involves the cleavage of carbon-carbon double bonds using ozone (O3). The reaction generates ozonide intermediates, which then undergo further transformations to produce specific carbonyl compounds.
- In the final steps, these ozonides are typically treated with reducing agents, such as zinc or dimethyl sulfide, leading to the formation of aldehydes, ketones, or carboxylic acids.
 Ozonolysis of Alkenes
Ozonolysis of Alkenes
Note:
Unbranched Alkene: Aldehyde
Branched Alkene: Ketone
From Alkyne
a. Kuchrov Reaction (Hydration of Alkyne):
- The hydration of an alkyne is a chemical reaction in which water is added to the carbon-carbon triple bond of an alkyne, resulting in the formation of a carbonyl compound. Specifically, the alkyne is converted into a ketone or aldehyde through this process.
- The reaction is typically catalyzed by acid, and the addition of water occurs across the triple bond, leading to the insertion of an oxygen atom.
 Kuchrov Reactionb. HydroBoration Oxidation
Kuchrov Reactionb. HydroBoration Oxidation
- Hydroboration-oxidation is a chemical reaction that involves the addition of boron and oxygen to an unsaturated organic compound, typically an alkene or alkyne. The process consists of two main steps.
- In the hydroboration step, the unsaturated compound reacts with boron compounds containing boron and hydrogen bonds. This results in the formation of a boron compound that is attached to the carbon atoms involved in the double or triple bond.
- In the oxidation step, the boron compound is treated with an oxidizing agent, usually hydrogen peroxide (H₂O₂) and sodium hydroxide (NaOH). This step replaces the boron group with an oxygen atom, resulting in the formation of aldehydes if the starting material is an alkene or ketones if it is an alkyne.
 HydroBoration Oxidation
HydroBoration Oxidation
Note: This reaction follows Anti-Markovnikov Addition.
Preparation of Aldehydes
Reduction of Acyl Halides, Esters and Nitriles
- The reduction of acyl halides, esters, and nitriles is a chemical transformation that leads to the formation of aldehydes.
- In these reactions, the carbonyl group of the acyl halide, ester, or nitrile is selectively reduced to an aldehyde functional group.
- Common reducing agents employed for these conversions include lithium aluminum hydride (LiAlH₄) or sodium borohydride (NaBH₄). The process involves the addition of hydrogen atoms to the carbonyl carbon, resulting in the conversion of the functional group.
 Reduction of Acyl Halide
Reduction of Acyl Halide Reduction of Esters
Reduction of Esters
 Reduction of Nitriles
Reduction of Nitriles
Rosenmund's Reduction
- The Rosenmund reduction is a chemical reaction that involves the partial reduction of a carboxylic acid derivative, typically an acid chloride, to an aldehyde using a catalyst such as palladium on barium sulfate.
- This method is useful for obtaining aldehydes selectively from more reactive acid chlorides, preventing further reduction to alcohols.
 Rosenmund's Reduction
Rosenmund's Reduction
Stephen's Reduction
- Stephen's reduction is a chemical method used to selectively reduce alkyl cyanides to aldehydes. It involves the reaction of alkyl cyanide with stannous chloride (SnCl2) and hydrochloric acid (HCl).
 Stephen's Reduction
Stephen's Reduction
Oxo Reaction(Hydroformylation)
- The Oxo Process is a significant industrial process for producing aldehydes from alkenes and synthesis gas (a mixture of carbon monoxide and hydrogen).
- Catalyzed by transition metal complexes, typically rhodium or cobalt, the reaction results in the addition of a formyl group (-CHO) to the alkene, forming aldehydes with a specific regioselectivity.
 Oxo Reaction
Oxo Reaction
Preparation of Aromatic Aldehydes
I) Oxidation of Methyl Benzene
Powerful oxidizing agents can turn toluene and its derivatives into benzoic acids through oxidation. However, it's feasible to halt the process at the aldehyde stage by using specific reagents. These reagents transform the methyl group into an intermediate that is resistant to further oxidation.
a. Etard Reaction:
The Etard reaction is a chemical reaction involving the oxidation of aromatic hydrocarbons, typically using chromyl chloride (CrO2Cl2) as the oxidizing agent.
 Etard Reaction
Etard Reaction
b. Use of Chromic Acid
- The chromic acid reduction of methylbenzene (toluene) involves the conversion of toluene to benzaldehyde.
- Chromic acid, often generated in situ from sodium dichromate (Na2Cr2O7) and sulfuric acid (H2SO4), serves as the oxidizing agent in this reaction.
 Chromic Acid Reduction of Toluene
Chromic Acid Reduction of Toluene
II) By side chain chlorination followed by hydrolysis
In this method, toluene is treated with chlorine, resulting in the formation of benzal chloride. When benzal chloride undergoes hydrolysis, it transforms into benzaldehyde.
 By side chain chlorination followed by hydrolysis caption
By side chain chlorination followed by hydrolysis caption
III) Gatterman Koch Reaction
The Gattermann-Koch reaction involves the reaction of an aromatic compound with carbon monoxide (CO) and hydrogen chloride (HCl) in the presence of a catalyst, usually aluminum chloride (AlCl3).
This process introduces a formyl group (-CHO) onto the aromatic ring, resulting in the formation of the desired aldehyde.
 Gatterman Koch Reaction
Gatterman Koch Reaction
Preparation of Ketones
From Dialkyl Cadmium
In this process, a dialkyl cadmium compound reacts with an acid chloride in the presence of a catalytic amount of titanium tetrachloride (TiCl4).
The reaction proceeds through the formation of an acyl-cadmium intermediate, which then undergoes rearrangement to produce the corresponding ketone.
 Preparation of Ketones from Dialkyl Cadmium
Preparation of Ketones from Dialkyl Cadmium
From Nitriles
Treating a nitrile with Grignard reagent followed by hydrolysis yields a ketone.
 Preparation of Ketones from Nitriles
Preparation of Ketones from Nitriles
From Benzene or Substituted Benzenes
- When benzene or a substituted form of benzene reacts with acid chloride in the presence of dry aluminum chloride, it produces the corresponding ketone.
- This chemical transformation is commonly referred to as the Friedel-Crafts acylation reaction.
 Preparation of Ketones from Benzene
Preparation of Ketones from Benzene
Uses of Carbonyl Compounds
- Propanone, a carbonyl compound, works as a solvent because it dissolves in both water and other organic solutions.
- Formaldehyde is employed in making plastics and is also used in biology labs for preserving specimens.
- Butanol is used to add fragrance to keep bread fresh.
- Acetaldehyde is a synthesizer in various organic reactions.
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|

|
Explore Courses for JEE exam
|

|



















