What are Optical Isomers? | Chemistry for JEE Main & Advanced PDF Download
What is Optical Isomerism?
Optical isomerism is a case where the isomers display identical characteristics in terms of molecular weight as well as chemical and physical properties. However, they differ in their effect on the rotation of polarized light.
- Optical isomerism occurs mainly in substances that have the same molecular and structural formula, but they cannot be superimposed on each other.
- In simple words, we can say that they are mirror images of each other. Alternatively, it can also be found in substances that have an asymmetric carbon atom.
 Depiction of Mirror Images of Alanine.
Depiction of Mirror Images of Alanine.
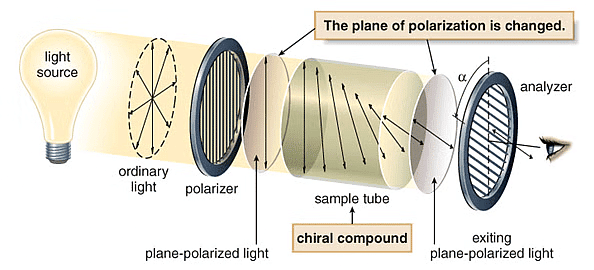
What is Plane Polarized Light?
If by filtering the beam with specialized materials, the electric field vectors are limited to a single plane, then the light is referred to as plane or linearly polarised with respect to the propagation direction. All waves vibrating in a single plane are referred to as plane-parallel or plane-polarized.
 Plane Polarized Light
Plane Polarized Light
- Optical isomerism is shown by stereoisomers which rotate the plane of polarized light.
- If the plane of polarized light passing through the enantiomer solution rotates in the clockwise direction then the enantiomer is said to exist as (+) form and if the plane of polarized light rotates in an anti-clockwise direction then the enantiomer is said to exist in (-).

- If the rotation of light is anti-clockwise then laevo rotatory (l-form)
- If the rotation of light is clockwise then the dextro rotatory substance(d-form)
- If there is no rotation of light then the substance is called optically inactive.
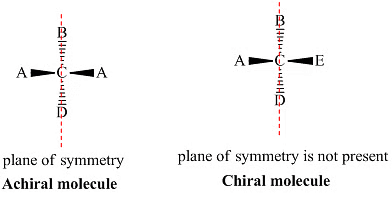
Chiral and Achiral Molecules
Chiral and achiral molecules are used to describe the symmetry characteristics of molecules. There are two main criteria to determine if a molecule is chiral or achiral:
- On the Basis of Atoms Attached
- On the Basis of Plane of Symmetry
- A chiral molecule is a molecule that is not superimposable on its mirror image. In other words, it does not possess an internal plane of symmetry.
- Achiral molecules are those that possess a plane of symmetry and are superimposable on their mirror image.
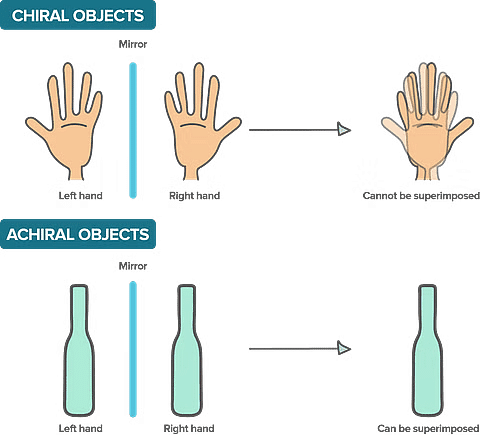 Superimposable and Nonsuperimposable Nature
Superimposable and Nonsuperimposable Nature
Chiral and achiral molecules can be identified based on two factors:
1. Based on Atoms Attached:
- If in a molecule, all the four valencies of carbon are satisfied by four different atoms or four different group atoms then carbon is known as chiral carbon and the molecule is called Chiral.
 Achiral and Chiral Compounds
Achiral and Chiral Compounds
- If in a molecule, all the four valencies of carbon are not satisfied by four different atoms or four different group atoms then carbon is known as achiral carbon and molecule is called Achiral.
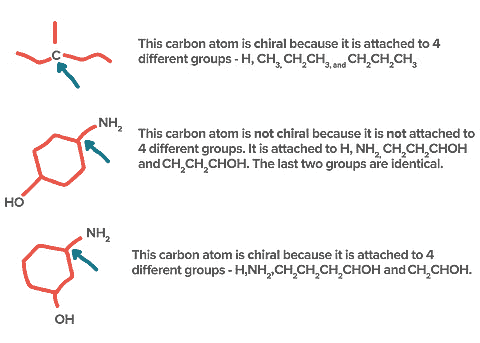 Identification of Chiral and Achiral CarbonsSome other Examples:
Identification of Chiral and Achiral CarbonsSome other Examples:
 2 Chiral Carbon
2 Chiral Carbon 2 Chiral Carbon
2 Chiral Carbon No Chiral Carbon
No Chiral Carbon
2. Based on Plane of Symmetry:
- If the molecule has a chiral center (four different groups are attached) and does not have a plane of symmetry or axis of symmetry then it is called a Chiral molecule.
- If the molecule has a plane of symmetry or axis of symmetry then it is called achiral molecule. Achiral molecules do not have a chiral center.
 Chiral and Achiral Molecule
Chiral and Achiral Molecule
Examples:
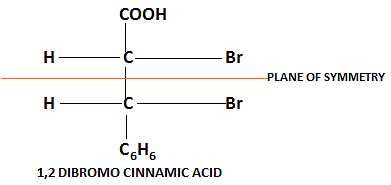 Achiral due to Plane of Symmetry
Achiral due to Plane of Symmetry
What are Enantiomers and Diastereomers?
Enantiomers and diastereomers are terms used to describe different types of stereoisomers, which are molecules that have the same molecular formula and connectivity but differ in their spatial arrangement.
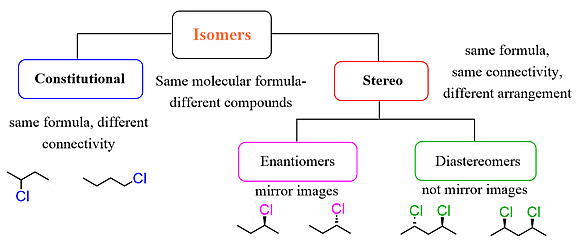 Flow Chart Explaining Enantiomers and Diastereomers
Flow Chart Explaining Enantiomers and Diastereomers
Enantiomers
Enantiomers are a pair of molecules that exist in two forms that can not be superimposed on each other but are mirror images of each other.
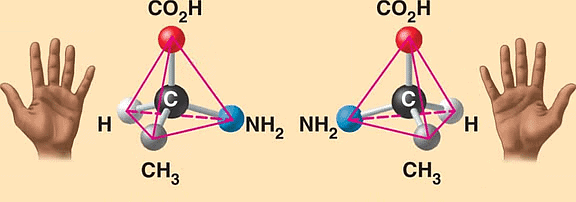 Enantiomers: Non-Superimposable Mirror Images
Enantiomers: Non-Superimposable Mirror Images
- Enantiomers are molecules with the same chemical formula and connectivity but differ in their three-dimensional arrangement. They cannot be superimposed on each other, similar to how our hands are mirror images but cannot be perfectly overlapped.
- Enantiomers share similar physical and chemical properties, but they exhibit different optical behavior. When plane-polarized light is passed through enantiomers, they demonstrate different optical properties, such as the direction and degree of rotation of the light.
- If we imagine placing a mirror between the two enantiomers and looking at one of them, we would see the other enantiomer reflected in the mirror. This is because the original enantiomer and its mirror image have distinct spatial arrangements and are considered enantiomers.
- It is important to note that if an object and its mirror image can be superimposed, they are not enantiomers. Enantiomers are specifically defined as non-superimposable mirror images.
However, if an object and its mirror image cannot be superimposed, they are optically active and referred to as enantiomers.
To determine if an object and its mirror image are superimposable, we can perform a test. Either the object or its mirror image is rotated by 180 degrees along with the mirror. After this rotation, we examine if the mirror image can be overlapped perfectly with the original object. Let us consider two examples:
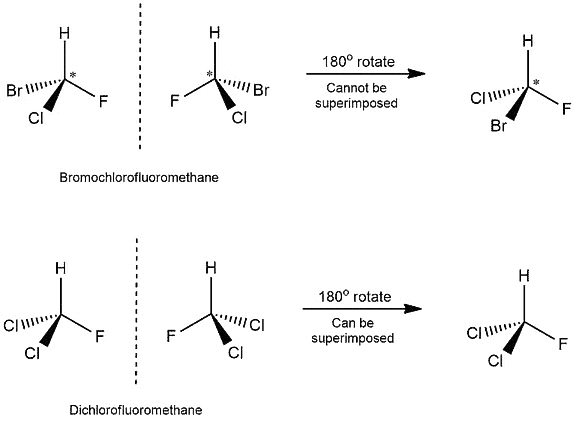 Identifying Superimposable Images
Identifying Superimposable Images
- In the first example, we can see that four different atoms are attached to the carbon atom. Those are bromine, chlorine, fluorine, and hydrogen. The chiral center is marked by a star (*), which is the usual way of marking it.
- When we would put the mirror next to the molecule on the left, we would get the molecule on the right. Then, if we would rotate this molecule by 180 degrees, we wouldn’t get the same molecule as on the left. Hydrogen and fluorine will be in the same position but chlorine and bromine on the opposite. And because of that, we get non-superimposable structures.
- In the other example, we have two chlorine atoms attached to the carbon. When we rotate the mirror image, we get the same molecule as on the left! This means that this molecule has a superimposable structure.
Diastereomers
A diastereomer is a stereoisomer with two or more stereocenters and the isomers are not mirror images of each other.
- If a molecule has more than one stereocenter and every single stereocenter isn’t in the opposite direction then they are not enantiomers, but diastereomers.
- In the following example, we have three stereocenters. On the first and third, the configuration is different (which would resemble the enantiomers) but on the second stereocenter is the same configuration. This means that they aren’t enantiomers but diastereomers.
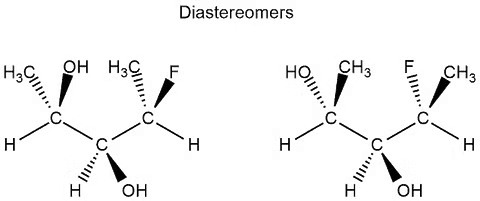 Example of Diastereomers
Example of Diastereomers
Example: Enantiomers and Diastereomers through Mirror Images:
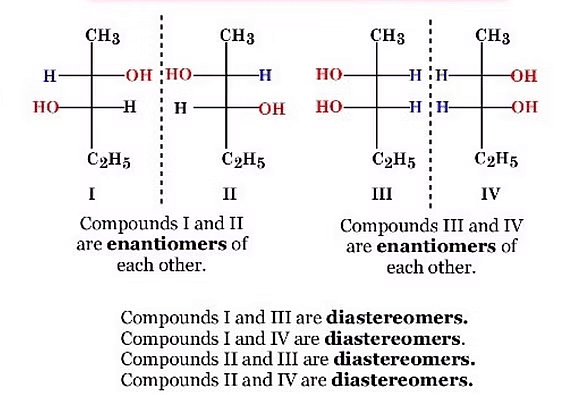 Identifying Enantiomers and Diastereomers through Mirror Images
Identifying Enantiomers and Diastereomers through Mirror Images
Some Solved Examples of Determining Enantiomers
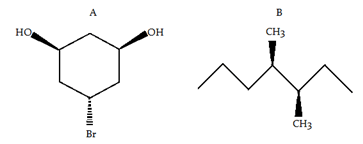
Q.1. Which of the following pairs of compounds is a pair of enantiomers?
A.
B.
C. 
D.

Solution: The correct option is B
- In option (d), one of the isomers has a plane of symmetry so it won't show chirality and hence, it cannot be its enantiomeric pair.
- Cyclohexane molecules exist in chair form and they do not have a plane of symmetry in the ring. Both the isomers in option (c) are not mirror images. Hence, they are not enantiomers.
- In option (a), both the structures are identical. They have the same configuration as the chiral centers.
 Identical Structures
Identical Structures - In (b), both are mirror images along the plane of paper and are not superimposable on each other. So, they are enantiomers.
Hence, (b) is correct.
Q.2. Which of the following is capable of existing as a pair of enantiomers?
A. 3-Methylpentane
B. 3-Methylhexane
C. 2-Methylpentane
D. 2-Methylpropane
Solution: 3- Methylhexane is optically active because of molecular asymmetry and chiral carbon. Hence it is capable of existing as a pair of enantiomers. 3-Methylhexane
3-Methylhexane
Determining Enantiomers and Diastereomers through R, S Assignments
The enantiomer of a molecule will always have an opposite R/S configuration. So to get the enantiomer all we need to do is flip all the stereocenters.
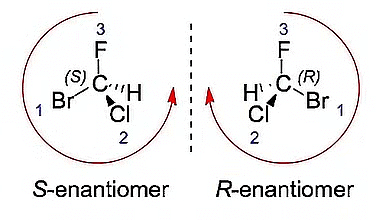 R-S Configuration of Enantiomers
R-S Configuration of Enantiomers
Diastereomers arise when at least two molecules share at least one (but not all) chiral center(s) with identical (R/S) configuration. So to find the diastereomers of (2R,3R,4R)-2,3,4,5-tetrahydroxypentanal, all we need to do is keep at least one stereocenter the same and flip any or all of the rest.
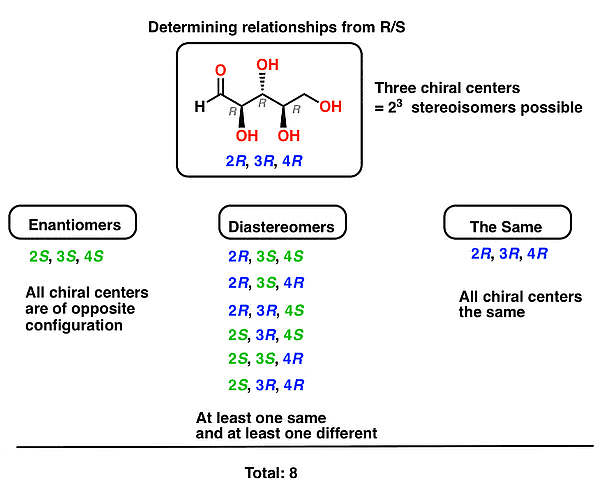 Determining Enantiomers and Diastereomers through R-S ConfigurationThe following rule can be applied to all molecules with two stereocenters:
Determining Enantiomers and Diastereomers through R-S ConfigurationThe following rule can be applied to all molecules with two stereocenters: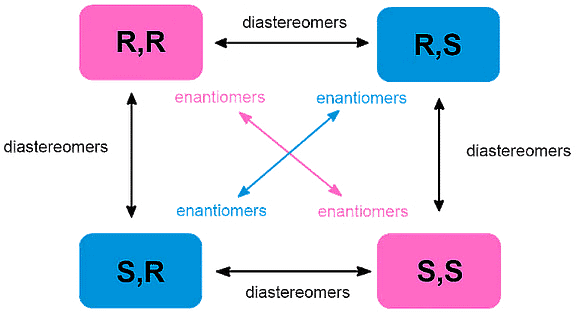
R-S configuration in Enantiomers and Diastereomers

The R and S designations, derived from the Cahn-Ingold-Prelog priority rules, are commonly used to differentiate enantiomers and establish their spatial configurations.
The Cahn Ingold Prelog (CIP) System For Naming Chiral Centers
Priority order:
- The Cahn Ingold Prelog (CIP) system is used to name chiral centers based on their priority.
- The groups around the chiral center are ranked from highest to lowest atomic number.
- We look at their atomic numbers, with the highest being priority 1 and the lowest being priority 4. If two groups have the same atomic number, we check the next atom's atomic number to determine priority.
- Few examples for assigning Priority Order:





To determine the configuration (R/S) of the chiral center:
- Position the chiral center so that the #4 priority substituent points away from you.
- Trace the path from priorities #1 to #2 to #3, ignoring #4.
- If the path traced is clockwise, the chiral center is assigned (R). If it is counterclockwise, the chiral center is assigned (S).
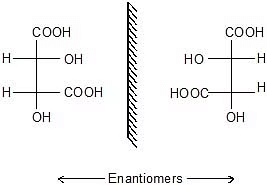
Racemic Mixture & Meso Compounds
There are organic compounds that have similar chemical formulas but different molecular structures. They are called enantiomers. When enantiomers are present in equal quantities in a mixture, it is called a racemic mixture.
- It is an equimolar mixture of R and S or d and l.
- A racemic mixture is optically inactive.
A compound is optically active due to:
(1) Absence of plane of symmetry (POS)
(2) Absence of centre of symmetry (COS)
- POS is an imaginary plane where if we place a mirror, the mirror image will exactly overlap the other half.
- For meso form, there must be at least two identical chiral carbons.
Identical carbon ⇒ Chiral carbons having identical groups attached. - If the compound has POS then it will be certainly optically inactive and will be called meso form.
 Meso Compound (POS Present)
Meso Compound (POS Present)
- After finding two identical carbons. We assign them as R or S. If the first part is R and the other is S then they will rotate the light in the opposite direction but to an equal extent the compound will be optically inactive. (meso form)
Allenes, Biphenyls, and Spiro Compounds
Allenes, biphenyls, and spiro compounds are interesting classes of organic compounds with unique structural features. Let's explore each of them:
1. Allene system

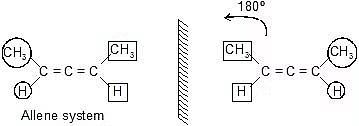
They are non-superimposable mirror image


Optically active
 Inactive
Inactive
2. Spiro Compounds


If no. of rings are even ⇒ optically active
If no. of rings are odd ⇒ Inactive


 Optically Active⇒ This is the even no. of double bonds case.
Optically Active⇒ This is the even no. of double bonds case.
For optical activity, the carbons at extreme positions must have different groups attached.
► 
⇒ Planar compound
Always have POS, Optically inactive.
3. Biphenyls

If biphenyl contains a bulky group at its ortho position (only) then due to repulsion the planarity of the compound disappears and its mirror image is non-superimposable.
► In the biphenyls none of the two rings must have symmetry.

Optically inactive
► 
Optically active
In 2º Amines.

Optically inactive due to the formation of a racemic mixture.
► Order of flipping in amines: 1º > 2º > 3º
Representation of Optical Isomers
Different types of representations can be used to depict the optical isomers on paper. There are mainly two types of representations of optical isomers:
- Wedge Dash
- Fischer Projection
Wedge Dash Representation
A Wedge Dash, the most popular three-dimensional depiction of a molecule on a two-dimensional surface is in projection (paper). This type of representation is typically used for molecules with chiral centres. This type of representation employs three different types of lines.

- Solid wedges or thick lines signify bond projections towards the observer or above the paper's surface. A continuous or regular line denotes a bond in the plane of the paper.
- A bond projection away from the observer is indicated by a dashed or broken line.
Fischer Projection
Fischer projections are best used to represent the straight-chain structures of monosaccharides and some amino acids. They represent structural forms that allow one to convey valuable stereochemical information by drawing 3D molecules as flat structures.
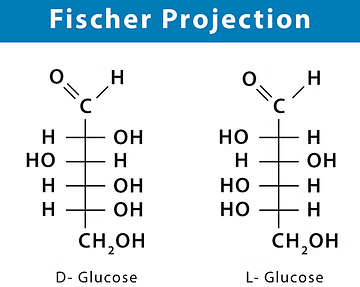 Fischer Projection
Fischer Projection
How to Draw Fischer Projection?
In a Fischer projection, the longest chain is drawn vertically. The horizontal lines indicate the bonds with hydrogen, hydroxyl, and amino groups. The four bonds to a chiral carbon make a cross, with the carbon atom at the intersection of the horizontal and vertical lines. The following steps can be employed for an aldohexose.
 Aldohexose
Aldohexose
Step 1: Arrange the molecule so that the chiral carbons and the longest continuous chain are in a vertical line. The aldehyde group representing carbon 1 goes at the top.
Step 2: Draw horizontal lines to make crosses at C-2, C-3, C-4, and C-5.
Step 3: Put the OH groups on the exact side of the cross.
Step 4: Remove C-2, C-3, C-4, and C-5, and the Fischer projection is obtained.
Fischer Projection Rules
The following rules should be kept in mind while working with Fischer projection.
- Fischer projection may be rotated by 180 degrees without changing its meaning.
- Fischer projection may not be rotated by 90 degrees. Such a rotation typically changes the configuration to the enantiomer.
- To find the enantiomer of a molecule drawn as a Fischer projection, exchange the right and left horizontal bonds.
Converting Wedge-Dash Structure to Fischer Projection
The stereochemical formula for (R)-lactic acid can be drawn using the wedge-dashed structure and Fischer projection method. The conversion from wedge-shaped or bond-line structure to Fischer projection is done stepwise.Examples:
Q.1. Write the Fischer projection of CH3CH(OH)COOH
Sol.

- Maximum carbon must be in a vertical line.
- Place higher priority carbon-containing functional group on top of the vertical line.
Q.2 Write Fischer's projection of

Sol.
(i) Place a higher-priority carbon-containing functional group on top of the vertical line.
(ii) Arrange another group according to its clockwise or anti-clockwise position w.r.t. group on the top.

Q.3. Convert the following wedge-dash figure in the Fischer projection. Sol.
Sol.
Q.4. Out of the two cross lines in the representation of Fischer projection, what does the horizontal line represent?
Ans: The Fischer Projection consists of both horizontal and vertical lines, where the horizontal lines represent the atoms that are pointed out of the plane while the vertical line represents atoms that are pointed away from the plane. The point of intersection between the horizontal and vertical lines represents the central carbon.
Q.5. Draw the fisher projection of:

Sol.
 *
* 
If fourth valency is not given then we assume it to be hydrogen.
Assigning R-S Configuration in Wedge Dash
 R → Rectus → Right → Clockwise.
R → Rectus → Right → Clockwise.
 S → Sinister → Left → Anti-clockwise.
S → Sinister → Left → Anti-clockwise.
(i) Determining R/S When The #4 Priority Substituent Is In Back (i.e. on a “Dash”)
When the #4 substituent is in the back (on a "dash"), follow the regular rules mentioned above. Here is an example:
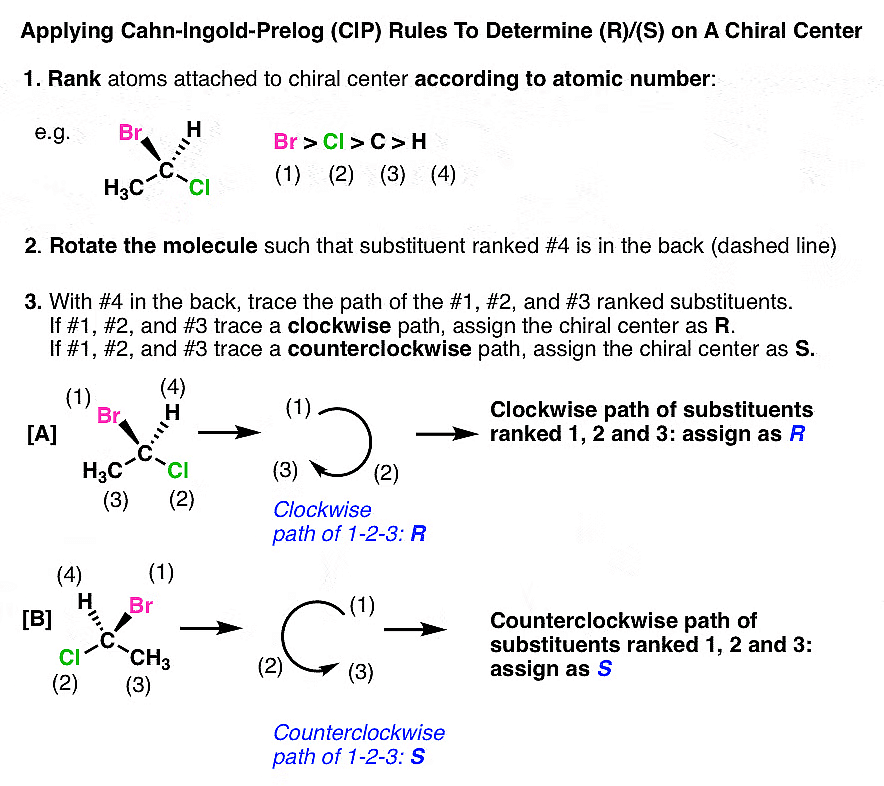
Note: Designations (R) and (S) bear no relationship to whether a molecule rotates plane-polarized light clockwise (+) or counterclockwise (-).
(ii) Determining R/S When The #4 Priority Substituent Is In Front (i.e. on a “Wedge”)
Let’s first consider the molecule below. The name of this molecule is (R)-1-fluoroethanol. It is listed below with priorities assigned based on atomic number. In this case F>O>C>H. So F is #1 and H is #4. The tricky part here is that the #4 priority is pointing out of the page (on a “wedge”).
You can “simply” rotate the molecule in your head so that the #4 priority is on a dash. Then you can traditionally assign R or S. This “simple” advice is not always an easy task for beginners.
 Rotating the Molecule
Rotating the Molecule
Here’s another way around this. When the #4 priority is on a wedge you can just reverse the rules. So now we have two sets of rules:
If the #4 priority is on a dash:
If the #4 priority is on a wedge, reverse the typical rules:
(iii) Determining R/S when the Lowest Priority Group is in the Plane of the Page
If the Lowest Priority Group group is in the plane of the page, swapping any two groups will change the configuration from R to S or vice versa. To determine the configuration:
- Swap the #4 substituent with the substituent in the back.
- Redraw the chiral center and determine R/S on the new configuration with the #4 group in the back.
- Flip the result to its opposite to account for the single swap made in step #1.
 When the Lowest Priority Group is in the Plane of Page
When the Lowest Priority Group is in the Plane of Page
Calculating the Total no. of Optically Active Isomers
1. If a compound has 'n' different chiral carbons then the total no. of optically active isomers = 2n
No. of meso form = 0
e.g.

no. of different chiral carbon = 4
total optical isomer = 2n = 24 = 16
- There will be no meso as the compound does not have identical chiral carbon.
- If a compound has n identical chiral centre (symmetrical) ⇒ There must be symmetry from somewhere.
Example: (Glucose)

Total no. of different chiral carbon = 4
Total Optical Isomers = 24 = 16
(i) If n is even
- number of optical isomers (a) = 2n-1
- meso forms (m) = 2n/2-1
- total number of optical isomers = a + m
(ii) If n is odd

Total optical isomers =  = 2n-1
= 2n-1
Example 1:

- When there is an odd no. of identical carbon atoms (i.e. symmetrical) then this compound will certainly contain pseudo chiral w.r.t. which compound is symmetrical (i.e. POS).
- Other meso compounds of the above compound will form by changing the place Br and H around pseudo-chiral carbon.

Total meso forms = 2
Total optical isomers = 
= 
= 22 - 2
= 4 - 2 = 2
Here are the two Optical Isomers:

Example 2:

Total no. of even chiral = 4
Number of optical isomers = a =  =
=  = 23 = 8
= 23 = 8
Meso forms = m =  =
=  = 22-1 = 21 = 2
= 22-1 = 21 = 2
Total number of optical isomers = 8 + 2 = 10
Diastereoisomers through Fischer Projection
For a single chiral center, there is no diastereoisomer. The stereoisomers which are not related as object and mirror images. They may be optically active or optically inactive.
 Inactive
Inactive ActiveFor a compound having 2 chiral centers, Fix one chiral carbon
ActiveFor a compound having 2 chiral centers, Fix one chiral carbon

After one inter-change, if (R, R) → (R, S), then we get diastereomers.
For a compound having 3 chiral carbon to get diastereoisomer, fix two chiral carbon and one interchange with the left carbon or fix one chiral carbon and interchange with other two,



(I) (II)
Total isomer = 23 = 8



(III) (IV)
(I) and (III), I and (IV), (II) and (III), (II) and (IV) are diastereoisomers.
Some Solved Examples
Q.1.
(I) 
(II) 
(III) 
(IV)  What is the relation among the above compounds?
What is the relation among the above compounds?
Sol. I and II are identical
III and IV are identical
II and III are diastereo isomer
I and IV are diastereo isomer
Q.2. Find the total isomers obtained by dichlorination of cyclopentane.
Sol.
Total isomers = 3 + 3 + 1 = 7
Optically isomers = 6, Optically active isomers = 4
Q.3. Find the total isomers obtained by trichlorination of propane.
Sol.


Total isomers = 6
optically isomers = 2
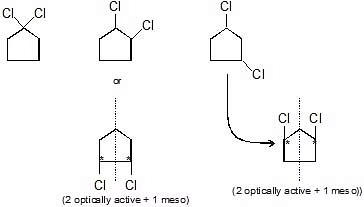
Q.4. Find total isomers obtained by dichlorination of n-butane
Sol.
 1
1
 2
2
 2
2
 1
1
 1
1
 3 (2 optically 1 meso ) Total isomers = 10 (6 optically active + 1 meso + 3 structural)
3 (2 optically 1 meso ) Total isomers = 10 (6 optically active + 1 meso + 3 structural)
Q.5. How many stereoisomers of 1,2,3-cyclohexantriol are there?
Sol.
 No. of Chiral carbon = 3 (identical) symmetrical)
No. of Chiral carbon = 3 (identical) symmetrical)
a =  =
=  = 4- 2
= 4- 2
m = 2
total stereoisomers = 2 + 2 = 4
 Meso
Meso

Mesoform is optically inactive due to internal compensation and racemic mixture is optically inactive due to external compensation.
Q.6. A and B are enantiomers of each other. The specific rotation of A is 20 º. Rotation of mixture of A and B = -5º What is the percentage of the racemic part?
Sol. x mol A, 1-x mol B
x × 20 + ( 1- x ) (-20) = -5
20 x - 20 + 20x = - 5
40 x = 15 ⇒ x = 3/8 = 0.375
moles of A = 3/8
moles of B =  =
= 
 moles of A and
moles of A and  moles of B will form racemic mixture.
moles of B will form racemic mixture.
Enantiomer excess or optical purity =  -
-  =
= 
Q.7.
 What is the rotation of the mixture?
What is the rotation of the mixture?
Sol. Rotation will be due to B only,
= 0.3 × (-20º)
= - 6º
► Chiral compound → optically active compound
Q.8. Which of the following compounds is Chiral (Optically active)?
(A)

(B)

(C) Both
(D) None
Ans. (D)
|
352 videos|596 docs|309 tests
|
FAQs on What are Optical Isomers? - Chemistry for JEE Main & Advanced
| 1. What is Optical Isomerism? |  |
| 2. What is Plane Polarized Light? |  |
| 3. What are Chiral and Achiral Molecules? |  |
| 4. What are Enantiomers and Diastereomers? |  |
| 5. How can you determine Enantiomers and Diastereomers through R, S Assignments? |  |
|
352 videos|596 docs|309 tests
|

|
Explore Courses for JEE exam
|

|