Systematic Analysis of Anions | Chemistry for EmSAT Achieve PDF Download
What is Qualitative Inorganic Analysis?
Qualitative inorganic analysis involves an analytical chemistry approach aimed at determining the elemental makeup of inorganic compounds using different reagents. Its primary emphasis lies in identifying ions within aqueous solutions, necessitating the conversion of substances in different states into an aqueous solution prior to experimentation. The processes for identifying ions (both cations and anions) within aqueous solutions are known as Cation Analysis and Anion Analysis.
Qualitative Analysis of Anions
Preliminary Tests
Some preliminary tests are done before going to the anion analysis.
(A) Physical Examination: Colour and Smell
The physical examination of the unknown salt involves the study of colour, smell and density. Characteristic smell helps to identify some anions such as acetate, sulphide etc.
(B) Dry Heating Test
This test is performed by heating a small amount of salt in a dry test tube. Quite valuable information can be gathered by carefully performing and noting the observations. On heating, some salts undergo decomposition, thus evolving the gases.
(C) Identification of Anions (Acid Radicals)
The identification of the radicals is first done on the basis of the preliminary tests. The Dry heating test is one of the preliminary tests performed earlier which may give some important information about the acid radical present. The other preliminary tests are based on the fact that:
- CO32-, S2-, NO2- and SO32- react with dil. H2SO4 to give out CO2, H2S, NO2 and SO2 gases respectively. These gases on identification indicate the nature of the anion present in the salt.
- Cl-,Br-,I-,NO3- and C2O42- and CH3COO- react with conc. H2SO4 but not with dil. H2SO4 to produce characteristic gases.
- SO42- and PO43- react neither with dil H2SO4 nor with conc. H2SO4. These are, therefore, identified by individual tests.
Thus, these anions may be identified by performing the following tests below:
(1) Dil. H2SO4 Tests
Treat a pinch of the salt with dil. H2SO4 and identify the gas evolved.
Chemical Reactions Involved in Dil.H2SO4 Test:
Dilute H2SO4 decomposes carbonates, sulphides, sulphites and nitrites in cold to give gases. These gases on identification indicate the nature of the anion present in the salt.
(a) Carbonate: On treating the solid carbonate, CO2 is given off in the cold with brisk effervescence.
CaCO3 + H2SO4 → CaSO4 + H2O + CO2↑
(b) Sulphide: Sulphides when treated with dil. H2SO4 give H2S gas.
ZnS + H2SO4 → ZnSO4 + H2S↑
(c) Sulphite: On heating solid sulphite with dil.H2SO4, SO2 gas is evolved
Na2SO3 + H2SO4 → Na2SO4 + H2O + SO2↑
(d) Nitrite: On treating solid nitrite with dil. H2SO4, nitric oxide (NO) gas is evolved which readly gives brown fumes of NO2 with the oxygen of the air. 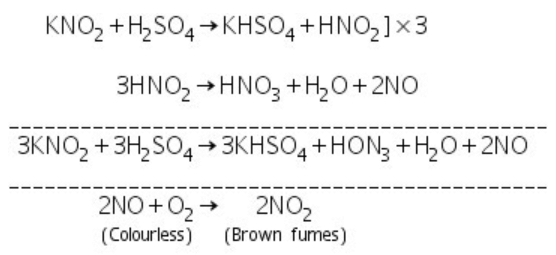
(2) Conc. H2SO4 Test
If no action takes place with dil. H2SO4, warm a pinch of the salt with conc. H2SO4 and identify the gas evolved.Chemical Reactions Involved in Conc. H2SO4 Test
(a) Chlorides: Chloride salts react with conc. H2SO4 to evolve hydrogen chloride (HCl) gas.

(b) Bromides: Bromide salts react with conc. H2SO4 to evolve bromine gas.
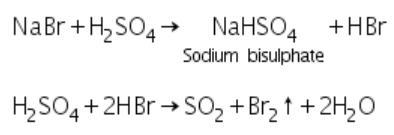
(c) Iodides: Iodide salts react with conc. H2SO4 to evolve vapours of iodine.
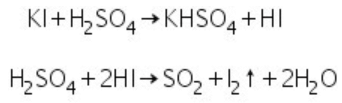
(d) Nitrates: Upon reaction with conc.H2SO4 nitrates evolve NO2 gas. 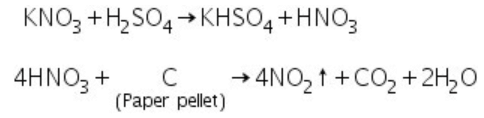
(e) Acetates: Acetates react with conc. H2SO4 to produce vapours of acetic acid.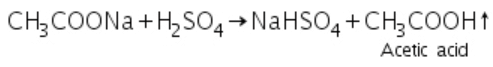
(f) Oxalates: Oxalates salts react with conc. H2SO4 to evolve a mixture of carbon dioxide and carbon monoxide. 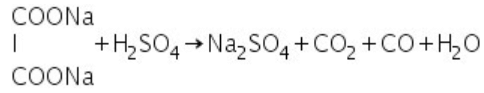
(3) Independent Group. (SO42- and PO43-) Test
If the salt does not react with dil H2SO4 as well as with conc. H2SO4 test for SO42-and PO43- by performing their individual tests.
(4) Potassium permanganate Test
This test is performed by using dilute sulphuric acid and potassium permanganate as reagents. This test helps in the detection of Cl-, Br-, I- and C2O42-.Chemical reactions involved in Potassium permanganate test
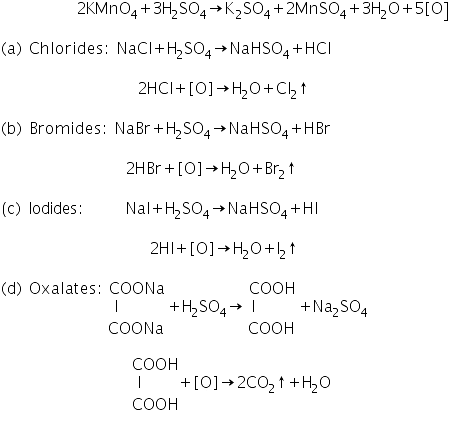
Wet Tests for Acid Radicals (Anions)
Let us discuss the chemical reactions involved in the confirmation of Anions:
Confirmation of Carbonate (CO32-)
(a) Reaction with di.l HCl
Carbonate on reaction with dil. HCl gives CO2 gas that reacts with lime water to produce a white precipitate of calcium carbonate that turns lime water milky. In case of soluble carbonate, this test is performed with water extract and in case of insoluble carbonates, this test is performed with the solid salt.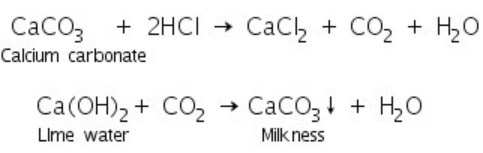
(b) Magnesium sulphate test
This test is performed in the case of soluble carbonates only. Soluble carbonates react with the magnesium sulphate solution to form a white precipitate of magnesium carbonate.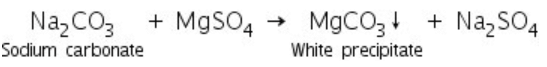
Confirmation of Sulphite (SO32-)
(a) Barium chloride test
Sulphites on reaction with barium chloride to form a white precipitate of barium sulphite. Barium sulphite dissolved in dil. HCl with the evolution of sulphur dioxide gas.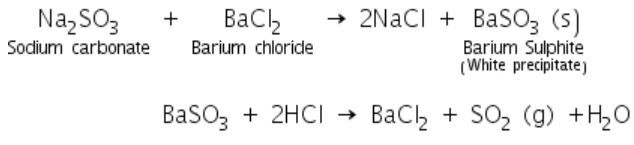
(b) Potassium permanganate test
The colour of potassium permanganate is discharged when it reacts with sulphite. The reaction is given below.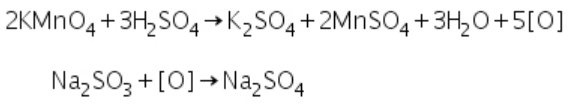
(c) Potassium dichromate test
A green colour is obtained when sulphites react with potassium dichromate solution.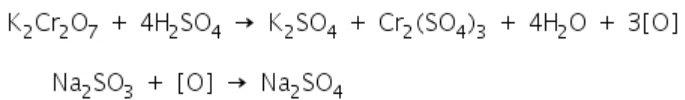
Confirmation of Sulphide (S2-)
(a) Sodium nitroprusside test
Sulphides give a violet or purple colouration with the sodium nitroprusside solution due to the formation of Na4[Fe(CN)5NOS].
(b) Lead acetate test
Sulphides react with lead acetate to form a black precipitate of lead sulphide.
(c) Cadmium carbonate test
Sulphides react with a suspension of cadmium carbonate to form a yellow precipitate of cadmium sulphide.
Confirmation of Nitrite (NO2-)
(a) Ferrous sulphate test
Nitrites give a dark brown or black colouration in Ferrous sulphate test due to the formation of FeSO4.NO.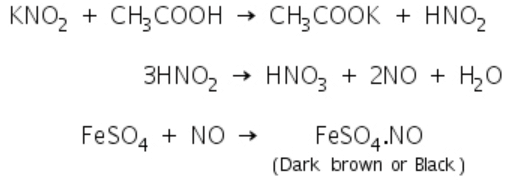
(b) Starch - Iodide test
Nitrites react with potassium iodide in the presence of dilute sulphuric acid to liberate iodine. Iodine forms a blue-black complex with starch.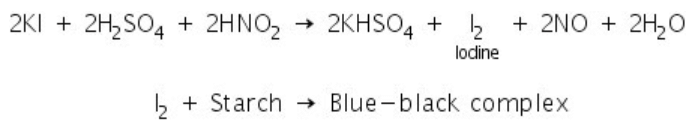
(c) Diphenylamine test
In the presence of nitrites, diphenylamine is oxidised, giving a blue colouration.
Confirmation of Nitrate (NO3-)
(a) Diphenylamine test
In the presence of nitrates, diphenylamine is oxidised, giving a blue colouration.
(b) Copper chips test
In this reaction copper chips reduces nitrates to reddish brown NO2 gas.
(c) Brown-ring test
This test can be performed by adding a solution of iron (II) sulphate to a solution of nitrate, followed by the slow addition of concentrated sulphuric acid, such that the sulphuric acid forms a layer below the aqueous solution. The formation of a brown ring at the junction of two layers indicates the presence of nitrate.
The overall reaction is the reduction of nitrate ion by iron (II) which reduced to iron (I) and formation of a nitrosonium complex where nitric oxide is oxidised to NO+.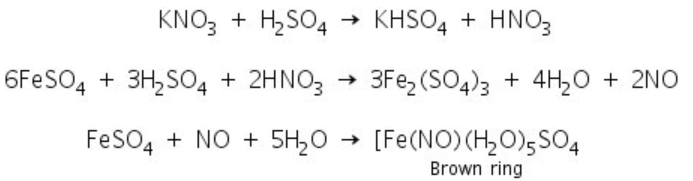
Confirmation of Chloride (Cl-)
(a) Silver nitrate test
Chlorides on reaction with silver nitrate solution to form a white precipitate of silver chloride which is soluble in ammonium hydroxide.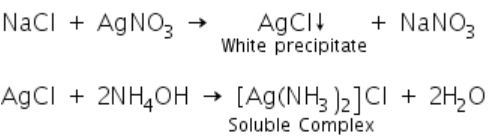
(b) Manganese dioxide test
When chloride salts react with manganese dioxide and concentrated sulphuric acid, chlorine gas is liberated.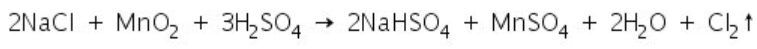
(c) Chromyl chloride test
When chloride salts react with potassium dichromate and conc. sulphuric acid red fumes of chromyl chloride is formed which reacts with sodium hydroxide to form yellow solution of sodium chromate. Sodium chromate reacts with lead acetate in presence of dil. acetic acid to form yellow precipitate of lead chromate.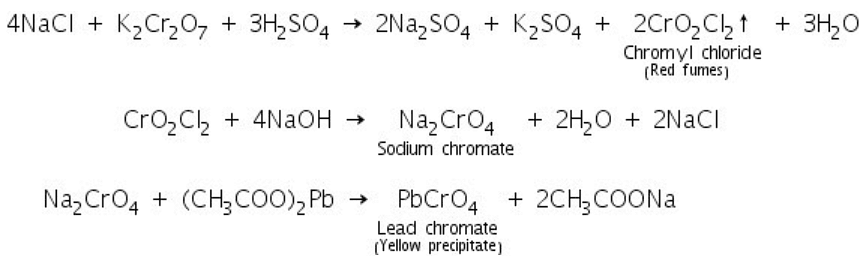
Confirmation of Bromide (Br-)
(a) Silver nitrate test
Bromides on reaction with silver nitrate solution forms a pale yellow precipitate of silver bromide which is sparingly soluble in ammonium hydroxide.
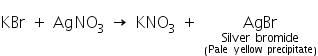
(b) Manganese dioxide test
When bromide salts react with manganese dioxide and concentrated sulphuric acid, bromine gas is liberated.

(c) Chlorine water test
Bromine liberated in this test being soluble in carbon disulphide imparts an orange colour to the carbon disulphide layer.
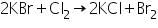
Note: Carbon tetrachloride, cyclohexane, chloroform etc can be used instead of carbon disulphide.
Confirmation of Iodide (I-)
(a) Silver nitrate test
Iodides on reaction with silver nitrate solution forms an yellow precipitate of silver iodide which is insoluble in ammonium hydroxide.
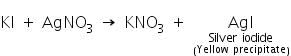
(b) Manganese dioxide test:
When iodide salts react with manganese dioxide and concentrated sulphuric acid, iodine gas is liberated.

(c) Chlorine water test
Iodine liberated in this test being soluble in carbon disulphide imparts a violet colour to the carbon disulphide layer.
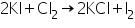
Confirmation of Acetate (CH3COO-)
(a) Oxalic acid test
Oxalic acid reacts with acetate salt to form acetic acid which has a characteristic vinegar like smell.
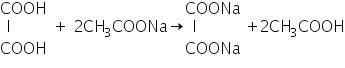
(b) Ester test
Acetate salts react with conc. sulphuric acid and ethyl alcohol to form the ester, ethyl acetate which has a fruity smell.
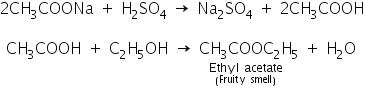
(c) Ferric chloride test
The reaction takes place in the ferric chloride test is given by the following equations.
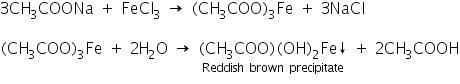
Confirmation of Oxalate (C2O42-)
(a) Calcium chloride test
Oxalate salts react with calcium chloride to form white precipitate of calcium oxalate.
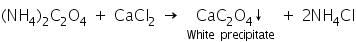
(b) Potassium permanganate test
In this test, the pink colour of potassium permanganate is decolourised with the evolution of carbon dioxide gas.
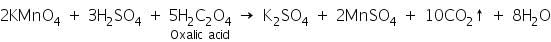
Confirmation of Sulphate (SO42-)
(a) Barium chloride test
Sulphates react with barium chloride to form white precipitate of barium sulphate.

(b) Match stick test
Violet streaks are produced during this test.
(c) Lead acetate test
Sulphates react with lead acetate to form white precipitate of lead sulphate.

Confirmation of Phosphate (PO43-)
(a) Ammonium molybdate test
Phosphate salts react with ammonium molybdate solution to form a deep yellow precipitate of ammonium phosphate molybdate. The chemical reaction is as follows:
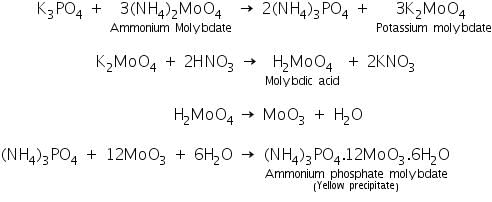
(b) Magnesia mixture test
Phosphate salts react with magnesia mixture to form white precipitate of magnesium ammonium phosphate.

|
198 videos|319 docs|164 tests
|
FAQs on Systematic Analysis of Anions - Chemistry for EmSAT Achieve
| 1. What is qualitative inorganic analysis? |  |
| 2. What does the qualitative analysis of anions involve? |  |
| 3. What are wet tests for acid radicals in qualitative inorganic analysis? |  |
| 4. How is systematic analysis of anions performed in qualitative inorganic analysis? |  |
| 5. How can EmSAT Achieve help with qualitative inorganic analysis? |  |




















