Protiens and Amino Acid | Zoology Optional Notes for UPSC PDF Download
What is an Amino acid?
- Amino acids are organic compounds essential for forming proteins, making them the building blocks of proteins.
- They play key roles in various biological and chemical functions in the human body.
- Necessary for human growth and development.
- Approximately 300 amino acids occur naturally.
- Characterized by containing amino groups (-NH2) and carboxyl groups (-COOH).
- Serve as the constituents of proteins.
- Both peptides and proteins consist of long chains of amino acids.
- A total of twenty amino acids are directly involved in protein synthesis.
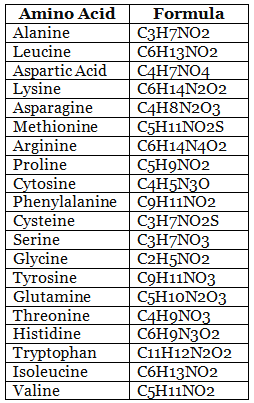
List of 20 Amino acids with the chemical formula
The twenty amino acids, along with their chemical formulas, are listed below.
General properties of Amino acids
- Amino acids possess high melting and boiling points.
- These compounds appear as white crystalline solids.
- Depending on the type, amino acids can taste sweet, be tasteless, or bitter.
- Generally, amino acids are water-soluble and insoluble in organic solvents.
Essential and Non-essential Amino acids
Non-Essential Amino Acids:
- Can be synthesized by the body.
- Include alanine, asparagine, arginine, aspartic acid, glutamic acid, cysteine, glutamine, proline, glycine, serine, and tyrosine.
Essential Amino Acids:
- Cannot be synthesized by the body and must be obtained through diet.
- Include isoleucine, histidine, lysine, leucine, phenylalanine, tryptophan, methionine, threonine, and valine.
Structure of Amino acids
General Structure of Amino Acids: Represented as H2NCH(R)COOH.
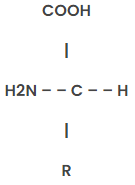
Common Features of the 20 Naturally Occurring Amino Acids:
- An amino group (-NH3+).
- A carboxylate group (-COO-).
- A hydrogen atom, all bonded to the same α-carbon atom.
- Variability comes from the side-chain, known as the R group, making each amino acid unique.
Four Distinct Groups Attached to the α-Carbon:
- Amino group.
- Carboxyl group (COOH).
- Hydrogen atom.
- Side-chain (R group).
Structure of 20 Amino acids with their chemical formula
Here is the structure of twenty amino acids with their chemical formula.
Sources of Amino acids
Roles of Amino Acids:
- Building and repairing tissues.
- Forming and functioning of enzymes.
- Assisting in food digestion.
- Transporting molecules.
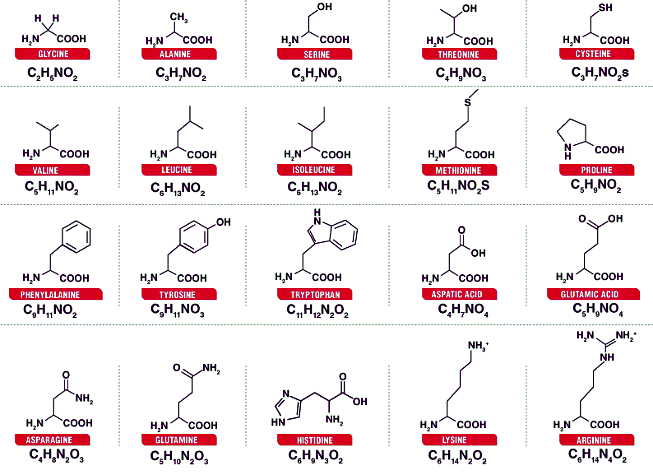
Sources of Amino Acids:
- Essential Amino Acids: Must be obtained through diet, found in protein-rich foods.
Plant-Based Foods High in Amino Acids:
- Broccoli, beans, beetroots, pumpkin, cabbage.
- Nuts, dry fruits, chia seeds, oats, peas.
- Carrots, cucumber, green leafy vegetables, onions.
- Soybeans, whole grains, peanuts, legumes, lentils.
Fruit Sources of Amino Acids:
- Apples, bananas, berries, figs, grapes.
- Melons, oranges, papaya, pineapple, pomegranates.
Animal Products Rich in Amino Acids:
- Dairy products, eggs, seafood.
- Chicken, meat, pork.
Functions of Amino acids
Functions of Essential Amino Acids:
- Phenylalanine: Supports a healthy nervous system and boosts memory power.
- Valine: Promotes muscle growth.
- Threonine: Enhances immune system functions.
- Tryptophan: Aids in producing vitamin B3 and serotonin, regulating appetite, sleep, and mood.
- Isoleucine: Crucial for hemoglobin formation, insulin synthesis, and oxygen transport.
- Methionine: Used in kidney stone treatment, skin health, and bacterial defense.
- Leucine: Stimulates protein synthesis and growth hormones.
- Lysine: Essential for antibody, hormone, enzyme formation, and calcium fixation in bones.
- Histidine: Participates in enzymatic processes and red and white blood cell synthesis.
Functions of Non-Essential Amino Acids:
- Alanine: Removes toxins and contributes to glucose and amino acid production.
- Cysteine: Acts as an antioxidant, vital for collagen production affecting skin texture and elasticity.
- Glutamine: Supports brain function and nucleic acid synthesis.
- Glycine: Important for cell growth, function, wound healing, and neurotransmission.
- Glutamic Acid: Functions as a neurotransmitter, crucial for brain development and function.
- Arginine: Aids in protein and hormone synthesis, detoxification, wound healing, and immune support.
- Tyrosine: Involved in thyroid hormone production, neurotransmitter and melanin synthesis.
- Serine: Supports muscle growth and immune protein synthesis.
- Asparagine: Facilitates nitrogen transport, DNA synthesis, nervous system development, and stamina improvement.
- Aspartic Acid: Vital for metabolism and synthesizing other amino acids.
- Proline: Important for tissue repair, collagen formation, preventing arteriosclerosis, and skin regeneration.
Deficiency of Amino acids
- Amino acids are crucial for protein synthesis, impacting nearly all biological processes.
- Consuming all nine essential amino acids daily is vital for healthy bodily functions.
- Amino acid deficiency can lead to various disorders, including:
- Edema.
- Anemia.
- Insomnia.
- Diarrhea.
- Depression.
- Hypoglycemia.
- Loss of appetite.
- Fat deposits in the liver.
- Problems related to skin and hair.
- Symptoms such as headache, weakness, irritability, and fatigue.
What is Protein?
Basic Composition of Proteins:
- Made up of amino acids.
- Contain carbon, hydrogen, oxygen, nitrogen, and sulfur.
Structure of Protein Molecules:
Large and complex, formed by twisted and folded strands of amino acids.
Roles of Proteins:
- Crucial in metabolism.
- Involved in movement.
- Key for defense mechanisms.
- Essential for cellular communication.
- Important in molecular recognition.
Functions of Proteins
Formation of Protein Structure:
- Positive and negative attractions between atoms in amino acid strands cause coiling into complex shapes.
- Folded proteins may join with others to create larger, more complex structures.
Impact of Protein Shape on Function:
- The specific folded shape of a protein determines its function in body chemistry.
Types of Proteins:
- Structural Proteins:
- Designed to form essential body structures.
- Example: Collagen provides tissue cohesion; Keratin forms waterproof fibers in skin's outer layer.
- Functional Proteins:
- Shapes allow participation in body's chemical processes.
- Include hormones, growth factors, cell membrane receptors, and enzymes.
Classification of Proteins
Protein Molecule Complexity:
- Composed of twisted and folded strands of amino acids.
- Amino acids are linked by covalent bonds.
Levels of Protein Structure:
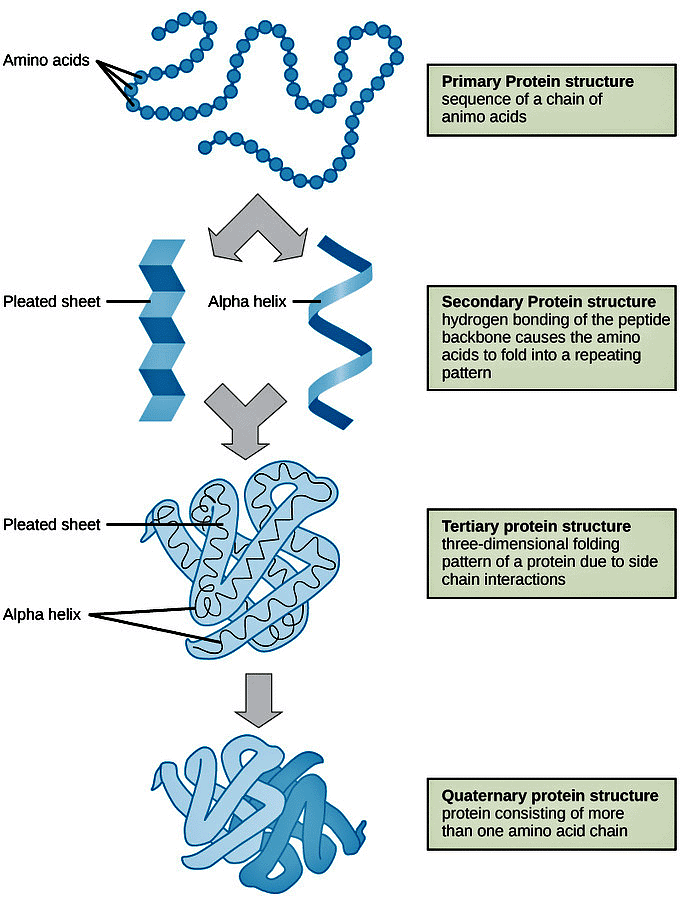
- Primary Structure: The sequence of amino acids in a chain.
- Secondary Structure: Formed by the folding and twisting of the amino acid chain.
- Tertiary Structure: Results from further folding of the secondary structure into a larger 3D structure.
- Quaternary Structure: Consists of more than one amino acid chain folded together.
Protein and Organic Compound Bonds:
Proteins can bind with other organic compounds to form mixed molecules, such as:
- Glycoproteins in cell membranes with attached sugars.
- Lipoproteins combining lipids and proteins.
Four Levels of Protein Structure
Importance of Protein Shape:
- Critical for protein function as it dictates the ability of the protein to interact with other molecules.
- Complex nature of protein structures required advanced techniques for accurate determination of their atomic-level structures.
Historical Development:
- Researchers historically faced challenges in determining complete protein structures due to the complexity and laborious nature of the techniques available, dating back to the 1950s.
- Recent advancements have facilitated easier and quicker determination of protein structures at the atomic level.
Four Levels of Protein Structure:
- Primary, Secondary, Tertiary, and Quaternary structures are conceptual divisions used to understand the complexity of protein structures.
- Understanding these levels is crucial for comprehending how proteins attain their final shape or conformation.
Primary Structure
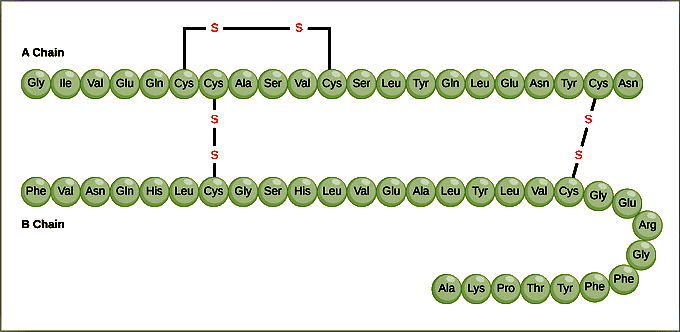
Definition of Primary Structure:- Represents the unique sequence of amino acids in each polypeptide chain that forms the protein.
- Essentially a linear list of amino acids in the order they appear in the chain.
Role of Primary Structure:
- Determines the final protein structure and function.
- Gene sequences, or DNA sequences, dictate the amino acid sequence in the polypeptide chain.
Impact of Gene Sequence Changes:
- Alterations in the nucleotide sequence of the gene's coding region can lead to different amino acids being incorporated into the polypeptide chain.
- These changes affect the protein's structure and function.
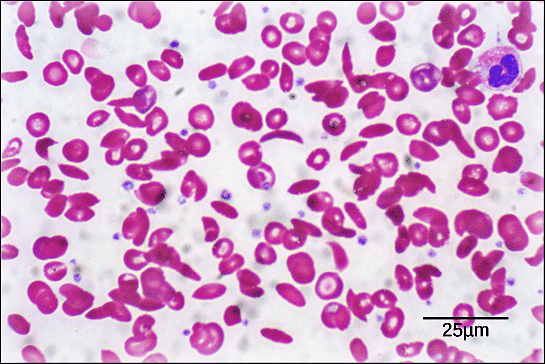
Example of Hemoglobin and Sickle Cell Anemia:
- Hemoglobin, an oxygen-transport protein, consists of four polypeptide chains: two α chains and two β chains.
- In sickle cell anemia, a single amino acid substitution in the hemoglobin β chain (glutamic acid to valine) causes a drastic change in protein structure.
- The altered hemoglobin forms dysfunctional proteins that aggregate under low-oxygen conditions, distorting red blood cells into sickle shapes and leading to various symptoms such as breathlessness, dizziness, headaches, and abdominal pain.
Secondary Structure
Definition of Secondary Structure:- Arises from interactions between neighboring or nearby amino acids as the polypeptide begins folding into its functional three-dimensional form.
Formation of Secondary Structures:
- Secondary structures develop as hydrogen bonds form between local groups of amino acids within a region of the polypeptide chain.
- Rarely does a single secondary structure extend throughout the entire polypeptide chain.
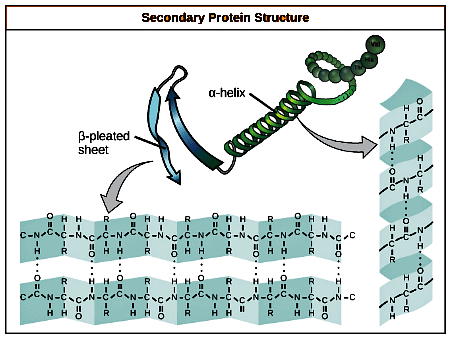
Common Secondary Structures:
- α-Helix: Forms a right-handed coil where hydrogen bonds occur between the oxygen atom in one amino acid and the hydrogen atom in another amino acid, four residues along the chain.
- Each helical turn consists of 3.6 amino acid residues.
- The R groups of amino acids protrude from the α-helix and do not participate in hydrogen bonding.
- β-Pleated Sheets: Composed of stretches of amino acids in an almost fully-extended conformation that zig-zags due to the non-linear nature of single C-C and C-N covalent bonds.
- β-Pleated sheets require the presence of other β-pleated sheets to maintain their structure.
- Hydrogen bonds form between the oxygen atom in one β-pleated sheet and the hydrogen atom in another β-pleated sheet.
- β-Pleated sheets align parallel or antiparallel to each other.
- The R groups of amino acids in a β-pleated sheet point outward perpendicular to the hydrogen bonds and do not contribute to the structure's stability.
Tertiary Structure
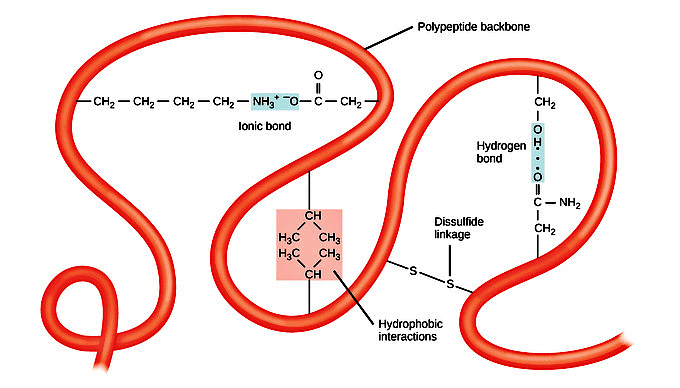
Tertiary Structure Definition:- Refers to the overall three-dimensional shape of a polypeptide chain.
- Formed after secondary structure elements fold together.
Interactions within Tertiary Structure:
- Involves interactions among polar, nonpolar, acidic, and basic R groups within the polypeptide chain.
- Polar interactions contribute to the complex tertiary structure.
Role of Environment:
- Hydrophobic R groups of nonpolar amino acids typically reside in the protein's interior in an aqueous environment.
- Hydrophilic R groups tend to be on the protein's surface.
Disulfide Linkages:
- Cysteine side chains form disulfide bonds in the presence of oxygen, the sole covalent bond during folding.
Overall Impact:
- Various interactions, both weak and strong, determine the final three-dimensional shape.
- Loss of this shape renders the protein non-functional.
Quaternary Structure
Quaternary Structure Definition:- Refers to the orientation and arrangement of subunits in multi-subunit proteins.
- Only applicable to proteins composed of more than one polypeptide chain.
Subunit Interactions:
- Weak interactions between subunits contribute to the protein's overall structural stability.
- Enzymes can be crucial in binding subunits to achieve the final protein structure.
Examples:
- Insulin, a globular protein, utilizes hydrogen and disulfide bonds to connect its two polypeptide chains.
- Silk, a fibrous protein, is formed by hydrogen bonding among β-pleated chains.
Key Points
Protein Structure Fundamentals:- Structure influenced by amino acid sequence and low-energy bonds in the backbone and side chains.
- Functional integrity dependent on maintaining specific structural levels.
Levels of Protein Structure:
- Primary Structure: Sequence of amino acids.
- Secondary Structure: Involves local interactions, forming α-helix and β-pleated sheet structures.
- Tertiary Structure: Overall 3D folding, mainly due to R group interactions.
- Quaternary Structure: Configuration of multiple subunits in a complex protein.
Key Terms
- Antiparallel: Refers to opposite orientations in DNA strands or beta strands in a protein’s secondary structure.
- Disulfide Bond: A covalent bond between two sulfur atoms from the reaction of two thiol groups, commonly between the thiol groups of cysteines in proteins.
- β-Pleated Sheet: A secondary protein structure where N-H groups in one strand's backbone form hydrogen bonds with C=O groups in an adjacent strand.
- α-Helix: A secondary protein structure where each N-H group forms a hydrogen bond with the C=O group of the amino acid four residues earlier within the same helix.
|
198 videos|351 docs
|
FAQs on Protiens and Amino Acid - Zoology Optional Notes for UPSC
| 1. What is an Amino acid? |  |
| 2. What are the essential and non-essential Amino acids? |  |
| 3. What are the general properties of Amino acids? |  |
| 4. What are the sources of Amino acids? |  |
| 5. What are the functions of Amino acids in the body? |  |
















