Grade 9 Exam > Grade 9 Notes > AP Chemistry > Changes in States
Changes in States | AP Chemistry - Grade 9 PDF Download
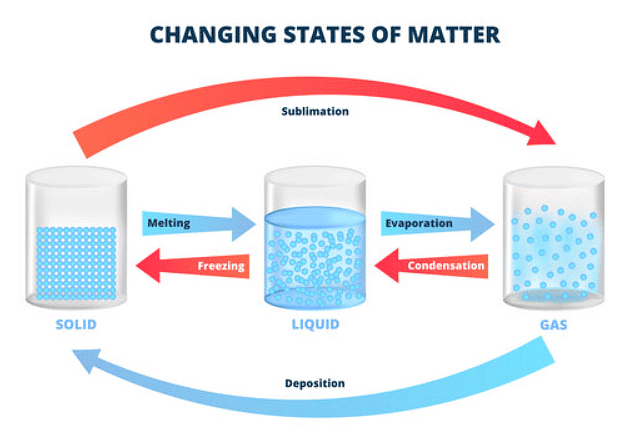
State Changes
Melting
- Melting refers to the process where a solid transforms into a liquid.
- It requires heat energy, which is converted into kinetic energy, enabling the particles to move freely.
- This transition occurs at a specific temperature called the melting point (m.p.).
Boiling
- Boiling is the transformation of a liquid into a gas.
- Heat is essential for this change, leading to the formation of gas bubbles beneath the liquid surface, allowing liquid particles to escape.
- Boiling transpires at a distinct temperature known as the boiling point (b.p.).
Freezing
- Freezing refers to the transformation of a liquid into a solid state.
- This process is the opposite of melting and happens at the same temperature as melting does. For instance, pure water freezes and melts at 0°C.
- It necessitates a considerable reduction in temperature (or loss of thermal energy) and takes place at a specific temperature.
Evaporation
- Evaporation is the change of a liquid into a gas and occurs at various temperatures.
- This phenomenon transpires exclusively at the surface of liquids, where high-energy particles can break free from the surface at low temperatures, typically below the liquid's boiling point.
- The rate of evaporation is influenced by the liquid's surface area and temperature, with larger surfaces and warmer temperatures leading to quicker evaporation.
Question for Changes in StatesTry yourself: What is the process by which a solid transforms into a liquid with the addition of heat energy?View Solution
Condensation
- Condensation is the process where a gas changes into a liquid upon cooling.
- This transition occurs over a range of temperatures.
- When a gas is cooled, its particles lose energy, causing them to group together and form a liquid.
Sublimation
- Sublimation is when a solid changes directly into a gas.
- This phenomenon is observed in specific solids like iodine or solid carbon dioxide.
- The reverse process, where a gas changes directly into a solid, is known as desublimation or deposition.
State Changes & Kinetic Theory
- When a substance is heated, its particles absorb thermal energy which is then converted into kinetic energy. This concept forms the basis of the kinetic theory of matter.
- Heating a solid leads to an increase in particle vibration. As the temperature rises, the particles vibrate more intensely, causing the solid to expand until its structure breaks and it melts.
- Upon further heating, a liquid substance expands further. Some particles at the surface gain enough energy to overcome intermolecular forces and evaporate.
- At the boiling point temperature, all particles acquire adequate energy to escape, resulting in the liquid boiling.
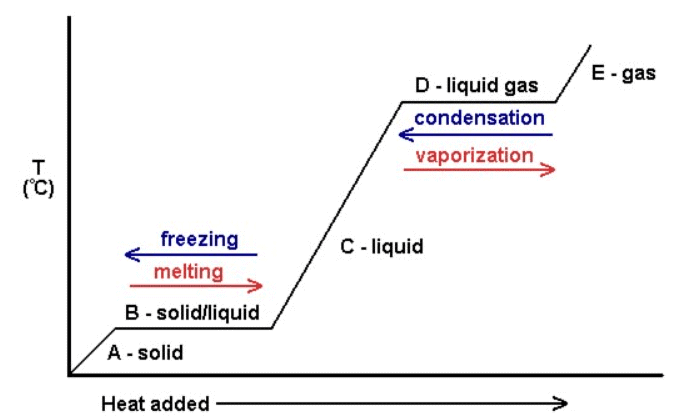
- State changes can be represented on a graph known as a heating curve.
- Cooling a gas has the opposite effect of heating and is depicted on a cooling curve.
- Cooling curves also show the impact of temperature changes on states of matter.
- The horizontal sections occur when there is a change of state but there is no change in temperature
Question for Changes in StatesTry yourself: What is condensation?View Solution
The document Changes in States | AP Chemistry - Grade 9 is a part of the Grade 9 Course AP Chemistry.
All you need of Grade 9 at this link: Grade 9
|
69 videos|92 docs|38 tests
|
FAQs on Changes in States - AP Chemistry - Grade 9
| 1. What are the four main state changes in matter? |  |
Ans. The four main state changes in matter are solid to liquid (melting), liquid to gas (vaporization), gas to liquid (condensation), and liquid to solid (freezing).
| 2. How does the kinetic theory explain state changes in matter? |  |
Ans. The kinetic theory states that matter is made up of particles in constant motion. When heat energy is added or removed, the particles move faster or slower, causing state changes such as melting, freezing, vaporization, and condensation.
| 3. What is the difference between evaporation and boiling in terms of state changes? |  |
Ans. Evaporation is the slow and gradual process of a liquid turning into a gas at temperatures below its boiling point, while boiling is the rapid and violent conversion of a liquid into a gas at its boiling point.
| 4. Why does a solid turn into a liquid when heated? |  |
Ans. When a solid is heated, the particles gain energy and start vibrating faster. This causes the particles to break free from their fixed positions and move more freely, resulting in the solid turning into a liquid.
| 5. How does pressure affect the state changes of matter? |  |
Ans. Pressure can affect the state changes of matter by altering the forces between particles. For example, increasing pressure can help keep particles closer together, making it harder for a substance to change states.
Related Searches
















