Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Effects of Temperature and Pressure on Volume of a Gas
Effects of Temperature and Pressure on Volume of a Gas | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Pressure & Temperature in Gases
- Changes in temperature or pressure impact how much space gases take up
- When the interior of a hot air balloon is heated, it enlarges, causing the balloon to increase in size
- This occurs because the volume of a gas rises with its temperature

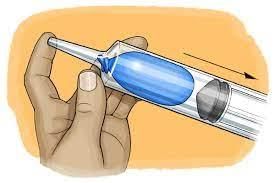
- When a gas is confined in a container and the volume is reduced through compression, the pressure of the gas increases.
- This phenomenon is akin to what occurs in a bicycle pump.
- By compressing the bicycle pump, the elevated pressure enables the inflation of a tire.
- The impact of this high pressure can be felt when placing a finger on the pump's end.
Question for Effects of Temperature and Pressure on Volume of a GasTry yourself: When the volume of a gas is reduced through compression, what happens to the pressure of the gas?View Solution
Gases and Kinetic Theory
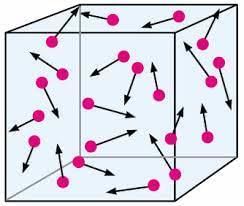
- Gaseous particles are in constant, random motion within a container.
- This motion generates pressure by the particles colliding with the container walls.
- Visualize molecules in a gas bouncing off each other and the container walls, creating pressure in the process.
- The pressure exerted by a gas is a result of the continuous, energetic movement of its particles.
- Kinetic Energy: When the temperature of a gas increases, the kinetic energy of its particles rises. For instance, think of a pot of water on a stove. As the heat is turned up, bubbles form at the bottom and rise to the surface faster.
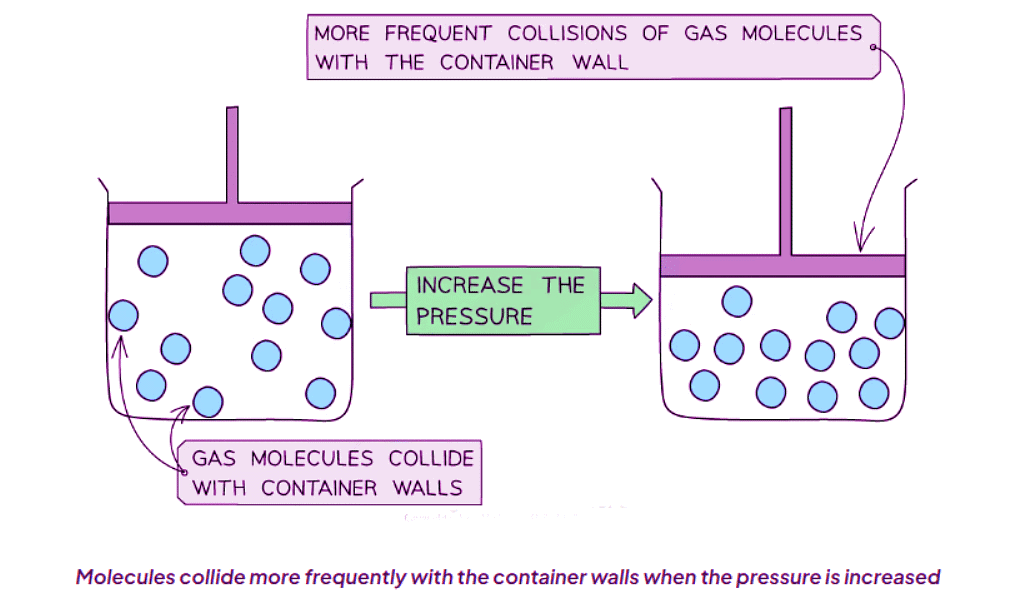
- Frequent Collisions: Imagine a balloon being inflated. As it expands, the rubber stretches. Similarly, when gas particles gain energy and move faster, they collide with the container walls more frequently, exerting pressure.
- Container Flexibility: A rubber balloon expands when filled with hot air, showcasing how flexible walls accommodate increased particle movement.
- Volume and Pressure: If you squeeze an air pump, reducing its volume, you will notice an increase in the pressure required to compress it further. This demonstrates the inverse relationship between volume and pressure in gases.
The document Effects of Temperature and Pressure on Volume of a Gas | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Effects of Temperature and Pressure on Volume of a Gas - Chemistry for GCSE/IGCSE - Class 10
| 1. How does temperature affect the volume of a gas? |  |
Ans. As temperature increases, the kinetic energy of gas particles also increases, causing them to move faster and collide more frequently with the container walls. This leads to an increase in pressure and therefore an increase in volume.
| 2. How does pressure affect the volume of a gas? |  |
Ans. When pressure is applied to a gas, the gas molecules are forced closer together, reducing the volume that the gas occupies. As pressure decreases, the gas molecules have more space to move around and the volume increases.
| 3. What is the relationship between temperature and pressure in a gas? |  |
Ans. According to the gas laws, as temperature increases, pressure also increases if the volume is kept constant. Similarly, if pressure increases, the temperature of the gas will also increase if the volume remains constant.
| 4. How do changes in temperature and pressure affect the behavior of gases? |  |
Ans. Changes in temperature and pressure can cause gases to expand or contract, change their density, and alter their ability to dissolve in liquids. These changes can impact various chemical reactions and physical properties of gases.
| 5. Can you provide an example of how temperature and pressure affect the volume of a gas in real life? |  |
Ans. One common example is a hot air balloon. When the air inside the balloon is heated, the temperature increases, causing the gas molecules to move faster and expand. This expansion increases the volume of the gas, making the balloon rise in the air.
Related Searches
















