Year 11 Exam > Year 11 Notes > Diffusion
Diffusion - Year 11 PDF Download
Diffusion
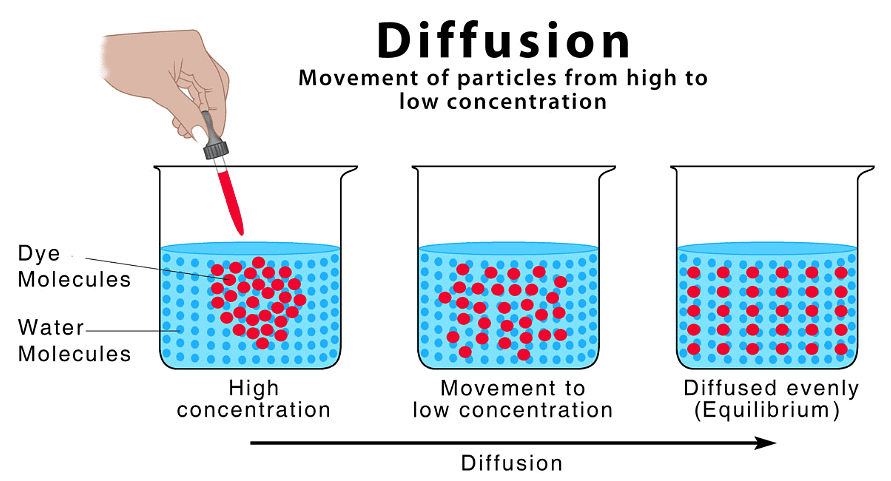
- This is the process by which different gases or different liquids mix and is due to the random motion of their particles.
- Diffusing particles move from an area of high concentration to an area of low concentration.
- Eventually the concentration of particles becomes even as they spread out to occupy all of the available space.
- Diffusion occurs spontaneously and does not require energy input, although it happens faster at higher temperatures.
Question for DiffusionTry yourself: What is diffusion?View Solution
Diffusion & Molecular Mass
- Diffusion happens more rapidly in gases compared to liquids because gas particles move at a higher speed than liquid particles.
- However, at the same temperature, different gases don't diffuse uniformly due to variations in their relative molecular masses.
- Lighter gas particles have the ability to travel faster and cover more distance, resulting in faster diffusion for gases with lower relative masses.
- This phenomenon can be observed in a reaction involving ammonia (NH3) and hydrogen chloride gas (HCl) in a lengthy glass tube.
- The formation of white smoke, representing ammonium chloride (NH4Cl), occurs closer to the end of the tube, contrary to the expected middle section, because hydrogen chloride (with a molecular mass of 36.5) and ammonia (with a molecular mass of 17) have smaller and lighter molecules.
Question for DiffusionTry yourself: Why does diffusion happen more rapidly in gases compared to liquids?View Solution
FAQs on Diffusion - Year 11
| 1. How does the molecular mass of a substance affect its rate of diffusion? |  |
Answer: The molecular mass of a substance directly affects its rate of diffusion. Generally, larger molecules have a slower rate of diffusion compared to smaller molecules because they have a harder time moving through the medium due to their size and weight.
| 2. Can diffusion occur in all states of matter? |  |
Answer: Yes, diffusion can occur in all states of matter - solid, liquid, and gas. In solids, diffusion is slower as the particles are closely packed, while in liquids and gases, diffusion is faster due to the higher mobility of particles.
| 3. How does temperature affect the rate of diffusion? |  |
Answer: An increase in temperature usually increases the rate of diffusion. This is because higher temperatures provide more kinetic energy to the particles, allowing them to move faster and diffuse more quickly.
| 4. How does concentration gradient impact the process of diffusion? |  |
Answer: The steeper the concentration gradient (difference in concentration between two areas), the faster the rate of diffusion. A larger difference in concentration means that particles will move more rapidly from an area of high concentration to an area of low concentration.
| 5. Can diffusion be affected by the size and shape of the particles involved? |  |
Answer: Yes, the size and shape of particles can impact the rate of diffusion. Smaller and less complex particles can diffuse more quickly compared to larger and more complex particles, as they encounter less resistance while moving through the medium.
Related Searches













