Periodic Table and Electronic Configuration | Chemistry for GCSE/IGCSE - Class 10 PDF Download
What is Electronic Configuration?
Electronic configuration
- We can represent the structure of the atom in two ways: using diagrams called electron shell diagrams or by writing out a special notation called the electronic configuration (or electronic structure or electron distribution)
- We can represent the structure of the atom in two ways: using diagrams called electron shell diagrams or by writing out a special notation called the electronic configuration (or electronic structure or electron distribution)
Electron shell diagrams
Electrons revolve around the nucleus in specific energy levels known as shells. Each shell possesses a distinct energy level, with the outer shells containing higher energy levels compared to the inner ones.
- Electrons tend to occupy the shell closest to the nucleus before filling up outer shells.
- When a shell reaches its electron capacity, any additional electrons start filling the subsequent shell.
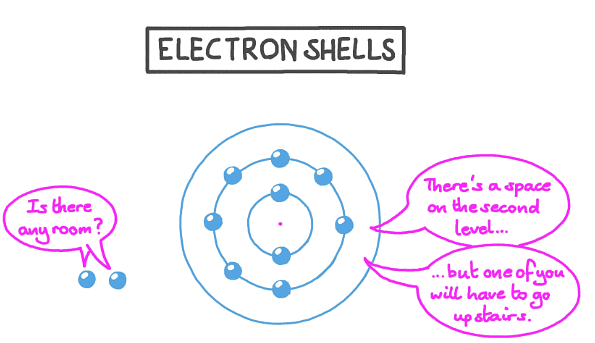
- The first shell has a maximum capacity of 2 electrons, while the second shell can accommodate up to 8 electrons.
- In a simplified model employed for introductory purposes, it is assumed that the third shell can hold 8 electrons.
- For the initial 20 elements, once the third shell attains its limit of 8 electrons, the fourth shell begins to populate.
- The outermost shell of an atom, known as the valence shell, plays a crucial role in determining the atom's stability.
- An atom is most stable when its valence shell is filled with the maximum number of electrons it can hold.
- The concept of electronic configuration simplifies the representation of electron arrangements in shells.
- Instead of using diagrams, we can express the number of electrons in each shell as a series of comma-separated values.
- This representation is known as electronic configuration or electronic structure.
- Take carbon, for instance, which possesses 6 electrons. Among these, 2 electrons occupy the first shell, while 4 electrons reside in the second shell.
- The electronic configuration of carbon is therefore denoted as 2,4.
- Take carbon, for instance, which possesses 6 electrons. Among these, 2 electrons occupy the first shell, while 4 electrons reside in the second shell.
- Electronic configurations are not limited to neutral atoms; they can also be assigned to ions.
- Consider sodium—an atom containing 11 electrons. When it loses one electron to become a sodium ion, it retains 10 electrons. In this ionized state, sodium has 2 electrons in the first shell and 8 in the second shell.
- Consequently, the electronic configuration of a sodium ion is represented as 2,8.
- Consider sodium—an atom containing 11 electrons. When it loses one electron to become a sodium ion, it retains 10 electrons. In this ionized state, sodium has 2 electrons in the first shell and 8 in the second shell.
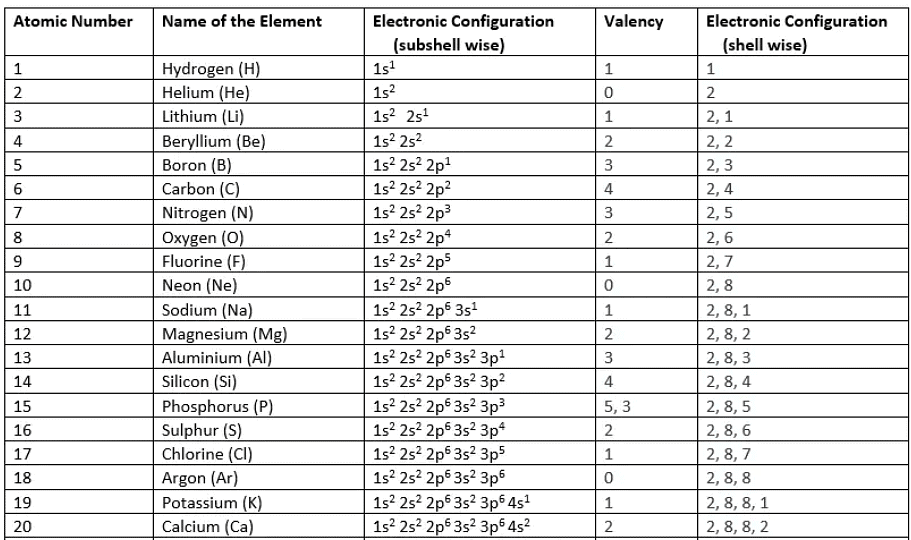
Note: After potassium and calcium, the filling of the electron shells becomes more complex. While the third shell can accommodate up to 18 electrons, for these elements, potassium and calcium, the third shell initially holds 8 electrons. The remaining electrons, for stability reasons, then occupy the fourth shell before filling the third shell.
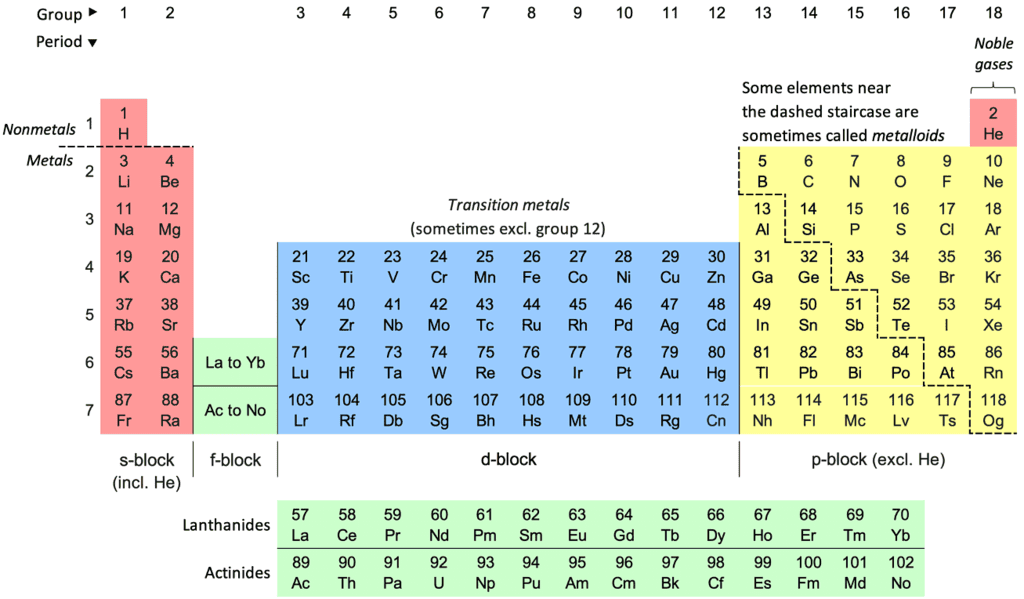
Electron Shells & The Periodic Table
- Concept: There is a significant link between an atom's electronic configuration and the structure of the Periodic Table.
- Explanation: The number of electron shells indicated in the electronic configuration corresponds to the period in the Periodic Table where the element is placed.
- Example: Sodium (Na) has an electronic configuration of 2.8.1, signifying it is in period 3 of the Periodic Table.
- Key Point: The final notation in the electronic configuration reflects the count of outer electrons and the group to which the element belongs (applicable to Groups I to VII).
- Insight: Elements in the same group possess an identical number of outer shell electrons.
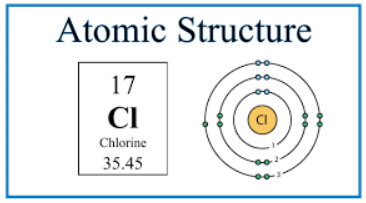
- Period: The red numbers at the bottom show the number of notations which is 3, indicating that a chlorine atom has 3 occupied shells of electrons and is in Period 3
- Group: The final notation, 7 in the example, indicates that a chlorine atom has 7 outer electrons and is in Group VII
- In most atoms, the outermost shell is not full. These atoms react with other atoms to achieve a full outer shell of electrons for stability.
- In some cases, atoms lose electrons to empty the outer shell, making the next shell below a full outer shell.
- All elements aim to fill their outer shells with electrons to attain a stable configuration.
The Noble Gases
- The atoms of the Group VIII elements (the noble gases) all possess a full outer shell of electrons.
- All noble gases demonstrate unreactivity due to their complete outer electron shells, rendering them highly stable.
|
71 videos|147 docs|61 tests
|
FAQs on Periodic Table and Electronic Configuration - Chemistry for GCSE/IGCSE - Class 10
| 1. What is electronic configuration and how is it related to the periodic table? |  |
| 2. How many electron shells are there in an atom and how do they affect the electronic configuration? |  |
| 3. How does the periodic table help in determining the electronic configuration of an element? |  |
| 4. Why is it important to understand electronic configuration in chemistry? |  |
| 5. Can the electronic configuration of an element change? |  |