Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Metallic Bonding
Metallic Bonding | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Metallic Bonding
- Metal atoms are strongly bound together through metallic bonding within a large metallic lattice.
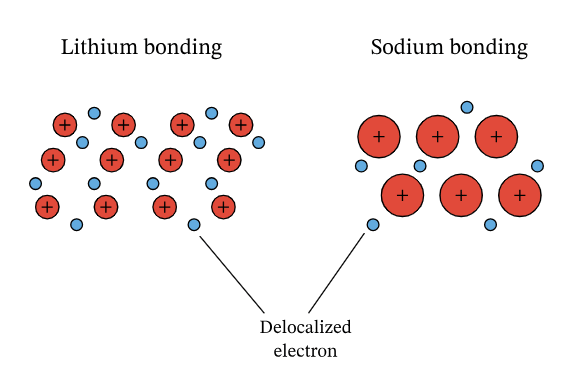
- In this lattice, atoms release electrons from their outer shell, transforming into positively charged ions.
- The outer electrons no longer belong to individual metal atoms but move freely, known as delocalisation.
- These delocalised electrons drift amidst the positive metal ions resembling a 'sea of electrons'.
- The strength of metallic bonds originates from the attractive forces between positive metal ions and the negatively charged delocalised electrons.
Question for Metallic BondingTry yourself: What is the main characteristic of metallic bonding?View Solution
Properties of Metals
Melting and Boiling Points
- Metals possess high melting and boiling points due to the presence of strong metallic bonds within their structures.
- Significant heat energy is necessary to break these bonds.
Electrical Conductivity
- Metals conduct electricity because of the availability of free electrons that can move through the structure to carry charge.
- When electrons enter one end of the metal, a delocalised electron displaces itself from the other end, facilitating the flow of electrons and the conduction of electricity.
Malleability and Ductility of Metals
- Positive ion layers have the ability to shift relative to one another, allowing for different arrangements.
- The integrity of metallic bonding remains intact because the outer electrons are not associated with specific metal atoms; consequently, the delocalized electrons move correspondingly.
- Due to this maintenance of metallic bonds, metals possess strength along with flexibility.
- Metals exhibit resilience, enabling them to endure processes like hammering, bending into diverse forms, or being drawn into wires without undergoing fracture.
Edurev Tip: When discussing the conductivity of metals, ensure clarity in the language employed and avoid confusion with ionic compounds. Metals conduct electricity due to the presence of unbound electrons capable of transporting charge, whereas molten or aqueous ionic compounds conduct electricity because they contain free ions that can carry charge.
Question for Metallic BondingTry yourself: What property of metals allows them to conduct electricity?View Solution
The document Metallic Bonding | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Metallic Bonding - Chemistry for GCSE/IGCSE - Class 10
| 1. What is metallic bonding? |  |
Ans. Metallic bonding is the type of chemical bonding that occurs between atoms in a metal. In metallic bonding, electrons are shared among a lattice of positively charged metal ions, creating a "sea of delocalized electrons" that hold the metal atoms together.
| 2. What are some properties of metals that are a result of metallic bonding? |  |
Ans. Some properties of metals that result from metallic bonding include high electrical and thermal conductivity, malleability, ductility, and luster.
| 3. How do metallic bonds differ from ionic or covalent bonds? |  |
Ans. Metallic bonds differ from ionic and covalent bonds in that they involve the sharing of electrons among a lattice of metal ions, rather than the transfer or sharing of electrons between specific atoms.
| 4. Can metals have different properties based on the strength of their metallic bonding? |  |
Ans. Yes, the properties of metals can vary based on the strength of their metallic bonding. Metals with stronger metallic bonds tend to have higher melting and boiling points, as well as greater hardness and strength.
| 5. How does the structure of a metal lattice contribute to its properties? |  |
Ans. The structure of a metal lattice, which consists of layers of closely packed metal ions surrounded by a sea of delocalized electrons, contributes to properties such as malleability, ductility, and the ability to conduct electricity and heat.
Related Searches




















