Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Chemical Formula of Compounds
Chemical Formula of Compounds | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Molecular Formulae
Element symbols
- Each element is represented by its own unique symbol as seen on the Periodic Table. For example, H represents hydrogen.
- Symbols consisting of two letters have the first letter capitalized and the second in lowercase. For instance, sodium is represented as Na, not NA.
- Atoms combine in fixed ratios to attain stable electron configurations. A chemical formula indicates these ratios. For instance, H2O shows two hydrogen atoms combining with one oxygen atom.
- By knowing the number of atoms in a molecule, you can deduce its chemical formula. For example, if a molecule has 3 hydrogen and 1 nitrogen atom, its formula is NH3.
- Diagrams or models can also represent chemical formulas. For instance, an ammonia molecule comprises a central nitrogen atom bonded to three hydrogen atoms.
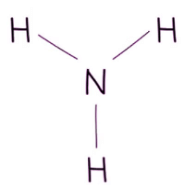
Chemical formulae
- The structural formula illustrates how atoms are bonded in a molecule, depicted through a diagram or written form. It provides a detailed arrangement of atoms within the compound.
- On the other hand, the molecular formula gives the actual count of each element's atoms present in one molecule of the compound. For instance, H2 signifies two hydrogen atoms, while HCl denotes one hydrogen atom and one chlorine atom.
Example: Butane
- Structural Formula (Displayed):
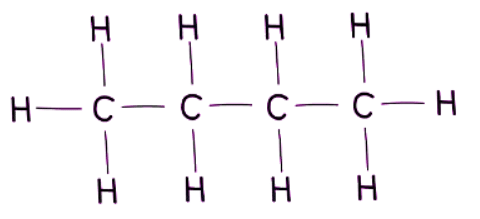
- Structural Formula (Simplified): Offers a condensed version of the structural formula in written form. Example: CH3CH2CH2CH3
- Molecular Formula: Indicates the number of each element's atoms in one molecule. For Butane, the molecular formula is C4H10.
- Empirical Formula: Represents the simplest whole number ratio of different atoms in a compound. The empirical formula for Butane is C2H5.
Question for Chemical Formula of CompoundsTry yourself: Which of the following best describes a molecular formula?View Solution
Deducing Formulae by Combining Power (Valency)
- The concept of valency is crucial in determining the formulas of compounds, whether they are molecular or ionic in nature.
- Valency, also known as combining power, indicates the number of bonds an atom can form with another atom or the number of electrons it can lose, gain, or share to create a compound. For instance, carbon belongs to Group IV, allowing a single carbon atom to establish 4 single bonds or 2 double bonds.
- The valencies associated with elements in various groups are as follows:
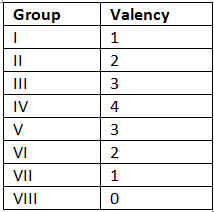
The document Chemical Formula of Compounds | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Chemical Formula of Compounds - Chemistry for GCSE/IGCSE - Class 10
| 1. What is the difference between molecular formulae and chemical formulae? |  |
Ans. Molecular formulae represent the actual number of atoms of each element present in a molecule, while chemical formulae give the types of atoms and their ratios in a compound.
| 2. How can one deduce formulae by combining power (valency)? |  |
Ans. By knowing the valency of each element, one can determine how many atoms of each element are needed to balance the charges and create a neutral compound.
| 3. Can you provide an example of deducing the chemical formula of a compound using valency? |  |
Ans. For example, in the compound sodium chloride (common salt), sodium has a valency of +1 and chlorine has a valency of -1. By combining them, the chemical formula becomes NaCl.
| 4. How can one write the chemical formula of compounds accurately? |  |
Ans. To write the chemical formula of a compound accurately, one must determine the valency of each element and balance the charges to create a neutral compound.
| 5. What is the significance of understanding molecular formulae in chemistry? |  |
Ans. Understanding molecular formulae is crucial in chemistry as it allows scientists to determine the composition of a compound and predict its properties and behavior in various reactions.
Related Searches
















