Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Relative Masses of Elements and Molecules
Relative Masses of Elements and Molecules | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Relative Atomic Mass
- Relative Masses: The symbol for the relative atomic mass is Ar: This symbol represents the relative atomic mass of an element.
- Locating Relative Atomic Mass: Each element's relative atomic mass can be found in the Periodic Table alongside its atomic number.
- Comparison of Masses: The relative atomic mass is typically greater than the atomic number, except for hydrogen, where they are equal.
- Comparing Atom Masses: Scientists faced challenges due to the small size of atoms, leading to the need for a method to compare their masses accurately.
- Standard Atom - Carbon-12: Carbon-12 is chosen as the standard atom with a fixed mass of 12 units for comparative purposes.
- Definition of Relative Atomic Mass (Ar): It is defined as the average mass of an element's isotopes in relation to 1/12th of the mass of a carbon-12 atom. Understanding relative atomic masses is fundamental in chemistry, aiding in the comprehension of various chemical phenomena and properties.
- Atomic Mass: The average mass of isotopes of an element is compared to 1/12 of the mass of an atom of carbon-12.
- Relative Atomic Mass: The relative atomic mass of carbon is 12, indicating that magnesium is twice as heavy as carbon. The relative atomic mass of hydrogen is 1, showing it has one-twelfth the mass of a carbon-12 atom.
Relative Molecular (Formula) Mass
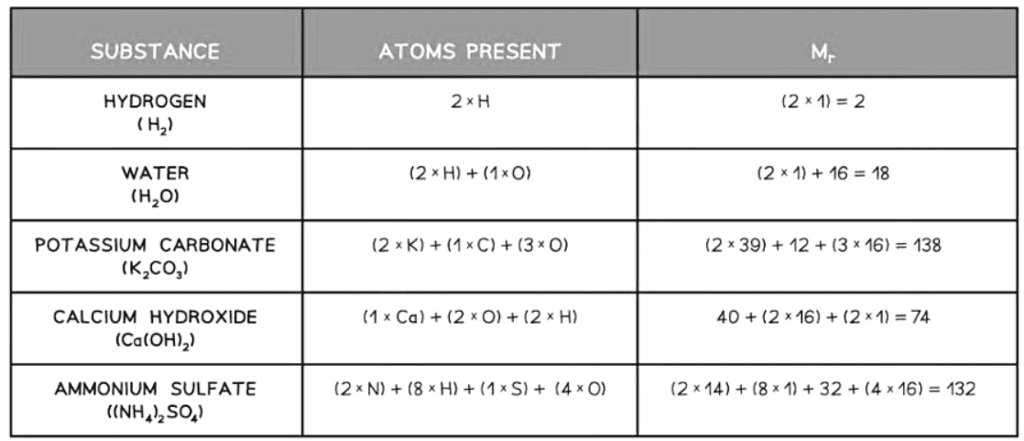
- The symbol for relative molecular mass is Mr, representing the total mass of a molecule.
- To determine the Mr of a substance, add the relative atomic masses of all atoms in the formula.
- Relative formula mass is utilized for the total mass of an ionic compound.
Relative Formula Mass Calculations Table
Question for Relative Masses of Elements and MoleculesTry yourself: What does the symbol "Ar" represent in chemistry?View Solution
Reacting masses
- The Law of Conservation of Mass states that mass remains constant and cannot be created or destroyed.
- In a chemical reaction, the combined mass of the reactants is equal to the combined mass of the products.
- Utilizing this law alongside relative atomic or formula masses enables us to calculate the quantities of reactants or products involved in a chemical reaction.
Example
- Given a chemical equation: 2Ca + O2 → 2CaO
- Considering the relative atomic masses: Ca = 40; O = 16
- By balancing the equation, we find that 2 atoms of calcium (2 x 40 = 80 units) react with 2 atoms of oxygen (2 x 16 = 32 units) to produce 2 x (40 + 16) = 112 units of CaO.
- This can be represented as: 2Ca + O2 → 2CaO
- Where 80 units of Ca combine with 32 units of O to form 112 units of CaO.
The Law of Definite Proportions
- The mass ratio of calcium and oxygen involved in a chemical reaction remains constant, regardless of the units used.
- For instance, 80 grams of calcium will always react with 32 grams of oxygen to produce 112 grams of calcium oxide. Similarly, 40 tonnes of calcium will react with oxygen to yield 56 tonnes of calcium oxide.
Stoichiometry in Chemical Reactions
- Stoichiometry principles dictate that the proportions in which substances react are fixed and predictable.
- For example, 80 grams of calcium will precisely combine with 32 grams of oxygen to form 112 grams of calcium oxide.
- Additionally, if 40 tonnes of calcium are exposed to excess oxygen, they will produce 56 tonnes of calcium oxide.
The document Relative Masses of Elements and Molecules | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
72 videos|162 docs|61 tests
|
FAQs on Relative Masses of Elements and Molecules - Chemistry for GCSE/IGCSE - Class 10
| 1. What is the difference between Relative Atomic Mass and Relative Molecular Mass? |  |
Ans. Relative Atomic Mass refers to the average mass of an atom of an element compared to 1/12th of the mass of a carbon-12 atom, while Relative Molecular Mass is the sum of the relative atomic masses of the atoms in a molecule.
| 2. How are Reacting Masses calculated in chemistry? |  |
Ans. Reacting Masses are calculated by using the balanced chemical equation to determine the ratio of moles of reactants and products, then converting these moles to grams using the molar mass of each substance.
| 3. How do you find the Relative Masses of Elements and Molecules? |  |
Ans. To find the Relative Mass of an element, you multiply the atomic mass of the element by the number of atoms present. For molecules, you add up the relative atomic masses of the atoms in the molecule.
| 4. Can you provide an example of calculating Relative Molecular Mass? |  |
Ans. For example, in the molecule H2O (water), the Relative Molecular Mass would be 1 (for hydrogen) + 1 (for hydrogen) + 16 (for oxygen) = 18.
| 5. Why is understanding Relative Atomic Mass and Molecular Mass important in chemistry? |  |
Ans. Understanding these concepts is crucial in determining stoichiometry, reaction yields, and predicting the behavior of substances in chemical reactions. It allows chemists to accurately calculate quantities involved in reactions and make informed decisions in experimental setups.
Related Searches















