Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Linking Moles, Mass and Mr
Linking Moles, Mass and Mr | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Calculating Moles
- In laboratories, chemicals react in specific ratios, but we measure them in grams using digital balances due to the impracticality of measuring moles directly.
- Conversion between moles and grams is essential.
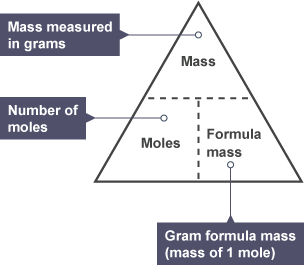
- The formula for converting moles, mass in grams, and molar mass is crucial.
- The molar mass represents the mass of one mole of a substance.
- For an element, the molar mass equals the relative atomic mass in grams.
- For compounds, the molar mass equals the relative formula or molecular mass in grams.
The Moles & Mass Formula Triangle
Question for Linking Moles, Mass and MrTry yourself: What is the formula for converting moles to grams?View Solution
The document Linking Moles, Mass and Mr | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
Related Searches
















