Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Percentage Yield
Percentage Yield | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Calculating Percentage Yield
- Yield refers to the quantity of product obtained from a chemical reaction.
- Achieving 100% yield in a chemical process is not feasible in practical scenarios due to various factors.
- These factors encompass:
- Residual reactants may remain in the apparatus after the reaction.
- Reversible reactions impede high yields as products revert to reactants continuously.
- Loss of products might occur during separation and purification procedures like filtration or distillation.
- Side reactions may transpire where a substance interacts with ambient gases or impurities within the reactants.
- Product losses can transpire during the transfer between containers.
Actual & Theoretical Yield
- Actual yield denotes the measured quantity of product acquired from a reaction.
- Theoretical yield represents the anticipated amount of product achievable under ideal practical and chemical circumstances, calculated based on the balanced equation and reactant masses.
- Percentage yield serves as a comparison between the actual yield and the theoretical yield.
- In pursuit of economic efficiency, chemical manufacturing companies aim for a high percentage yield to enhance profits, minimize expenses, and mitigate waste.
- The formula for calculating percentage yield is as follows:
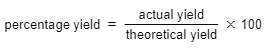
Calculating percentage mass
- Sometimes, you might need to determine the percentage by mass of an element in a compound.
- This involves computing the relative formula mass of the compound.
- The calculation requires using the following equation:

Calculating Percentage Purity
- Pure substances contain no other substances mixed with them.
- However, the product you aim to obtain might become tainted with undesirable substances like unreacted reactants, catalysts, or impurities.
- To determine the percentage purity, you use the following equation:

Question for Percentage YieldTry yourself: What is the purpose of calculating percentage yield in chemical processes?View Solution
The document Percentage Yield | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
72 videos|162 docs|61 tests
|
FAQs on Percentage Yield - Chemistry for GCSE/IGCSE - Class 10
| 1. How do you calculate percentage yield in chemistry? |  |
Ans. Percentage yield in chemistry is calculated by dividing the actual yield by the theoretical yield, then multiplying by 100 to get the percentage. The formula is: Percentage Yield = (Actual Yield / Theoretical Yield) x 100.
| 2. What factors can affect the percentage yield of a chemical reaction? |  |
Ans. Factors that can affect the percentage yield of a chemical reaction include the purity of the reactants, the efficiency of the reaction conditions, side reactions, and loss of product during purification.
| 3. How do you improve the percentage yield of a chemical reaction? |  |
Ans. To improve the percentage yield of a chemical reaction, one can increase the purity of the reactants, optimize reaction conditions, use catalysts to increase reaction efficiency, and minimize loss of product during purification.
| 4. What is the significance of calculating percentage yield in chemistry experiments? |  |
Ans. Calculating percentage yield in chemistry experiments helps determine the efficiency of a reaction, assess the quality of the experimental procedure, and make adjustments to improve future reactions.
| 5. How does calculating percentage yield help in real-world applications of chemistry? |  |
Ans. Calculating percentage yield in real-world applications of chemistry helps industries optimize production processes, reduce waste, and ensure cost-effective production of chemicals and materials.
Related Searches















