Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Electrolysis
Electrolysis | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Electrolysis: General Principles
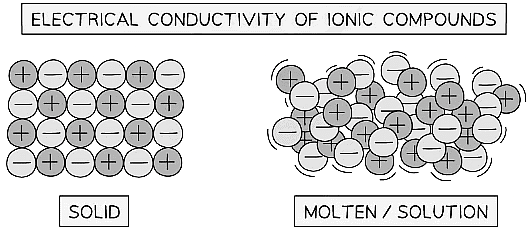
- When an electric current flows through a molten ionic compound, or through its aqueous solution, the compound undergoes decomposition.
- This decomposition process also applies to aqueous solutions of ionic compounds.
- Unlike covalent compounds, which cannot conduct electricity, ionic compounds do not undergo electrolysis.
- Ionic compounds in their solid state also cannot conduct electricity because they lack free ions capable of movement to carry the electrical charge.
Key Terms in a Simple Electrolytic Cell
- Electrode: A metal or graphite rod enabling electric current flow in or out of an electrolyte.
- Electrolyte: An ionic compound in molten or dissolved form that conducts electricity.
- Anode: The positive electrode in an electrolysis cell.
- Anion: A negatively charged ion attracted to the anode.
- Cathode: The negative electrode in an electrolysis cell.
- Cation: A positively charged ion drawn towards the cathode.
- The Basic Setup of an Electrolytic Cell
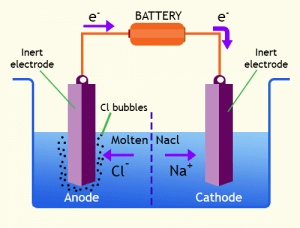
- Metals and hydrogen ions become positively charged ions, leading to the formation of either a metal or hydrogen gas at the cathode.
- Non-metals, except hydrogen, produce negatively charged ions, resulting in the formation of non-metallic substances at the anode.
Question for ElectrolysisTry yourself: What is the purpose of an electrode in an electrolytic cell?View Solution
Electrolysis: Charge Transfer
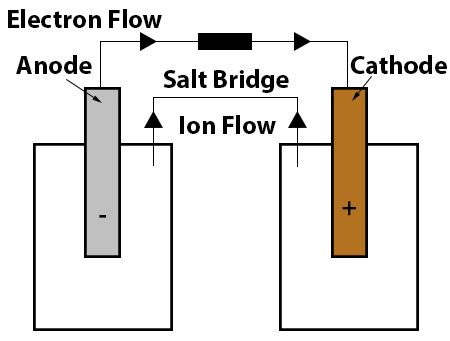
- When conducting electrolysis, an electric current must circulate around the circuit.
- For this process to take place, charge transfer occurs throughout the circuit by charge carriers.
- The power source supplies the cathode with electrons, leading it to acquire a negative charge.
- Positive ions, known as cations, within the electrolyte migrate towards the cathode where they acquire electrons.
- Negative ions, or anions, within the electrolyte move towards the anode where they release electrons.
- Electrons travel from the anode back to the power source.
- Within a complete circuit, electrons function as the charge carriers in the external circuit, while ions serve as the charge carriers in the electrolyte.
The document Electrolysis | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Electrolysis - Chemistry for GCSE/IGCSE - Class 10
| 1. What are the general principles of electrolysis? |  |
Ans. Electrolysis is a process where electrical energy is used to drive a non-spontaneous chemical reaction. It involves the transfer of charge, usually in the form of ions, at the electrodes in an electrolytic cell.
| 2. What are the key terms in a simple electrolytic cell? |  |
Ans. Some key terms in a simple electrolytic cell include electrodes (anode and cathode), electrolyte (solution or molten compound being electrolyzed), and electric current.
| 3. Why is charge transfer important in electrolysis? |  |
Ans. Charge transfer is important in electrolysis because it is the mechanism by which ions are oxidized at the anode and reduced at the cathode. This process is essential for the overall electrolysis reaction to occur.
| 4. How does electrolysis differ from galvanic cells? |  |
Ans. Electrolysis involves the use of electrical energy to drive a non-spontaneous chemical reaction, while galvanic cells produce electrical energy from a spontaneous redox reaction. In electrolysis, an external power source is needed, whereas galvanic cells operate spontaneously.
| 5. Can you provide an example of a practical application of electrolysis? |  |
Ans. One practical application of electrolysis is in the production of metals such as aluminum and sodium. Electrolysis is used to extract these metals from their ores through the reduction of their respective ions at the cathode.
Related Searches















