Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Electrolysis of Aqueous Sodium Chloride and Dilute Sulfuric Acid
Electrolysis of Aqueous Sodium Chloride and Dilute Sulfuric Acid | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Aqueous sodium chloride
- Brine is a concentrated solution of aqueous sodium chloride
- It can be electrolyzed using inert electrodes made from platinum or carbon/graphite
- During electrolysis, gas bubbles are produced at both electrodes, resulting in the formation of chlorine and hydrogen, with sodium hydroxide solution left behind
- These substances have significant industrial applications: Chlorine for bleach production, hydrogen for margarine production, and sodium hydroxide for soap and detergent manufacturing
Uses of Chlorine, Hydrogen, and Sodium Hydroxide
- Chlorine is utilized in the production of bleach.
- Hydrogen is essential in the manufacturing of margarine.
- Sodium hydroxide is a key component in the creation of soaps and detergents.
Products at the Electrodes during Electrolysis of Brine
At the Negative Electrode
- Hydrogen ions (H+) are discharged at the cathode due to their lower reactivity compared to sodium ions.
- These H+ ions accept electrons, forming hydrogen gas.
At the Positive Electrode
- Chloride ions (Cl-) are discharged at the anode.
- Upon losing electrons, chlorine gas is produced.
- Sodium (Na+) and hydroxide (OH-) ions remain, yielding sodium hydroxide (NaOH) solution.
Diagram showing the products of the electrolysis of aqueous sodium chloride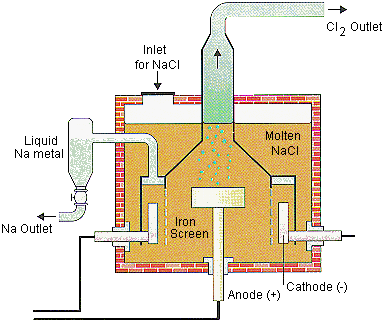
Question for Electrolysis of Aqueous Sodium Chloride and Dilute Sulfuric AcidTry yourself: What are the products formed at the negative and positive electrodes during the electrolysis of aqueous sodium chloride?View Solution
Electrolysis of Dilute Sulfuric Acid
- Dilute sulfuric acid can undergo electrolysis using inert electrodes like platinum or carbon/graphite.
- Bubbles of gas are observed at both the cathode and anode.
Product at the Negative Electrode
- Hydrogen ions (H+) migrate to the cathode, gain electrons, and produce hydrogen gas.
Product at the Positive Electrode
- Hydroxide ions (OH-) are attracted to the anode, lose electrons, and yield oxygen gas along with water.
- To determine the gas produced, if the gas at the anode reignites a glowing splint, then it's confirmed to be oxygen gas.
Diagram showing the test for oxygen gas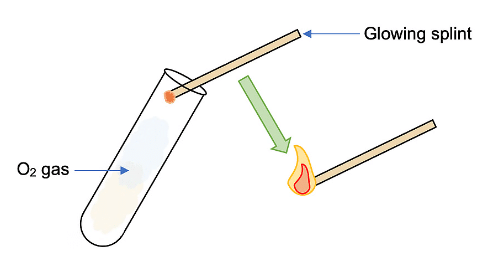
- If the gas produced at the anode bleaches damp litmus paper, then the gas is chlorine.
Diagram showing the test for chlorine gas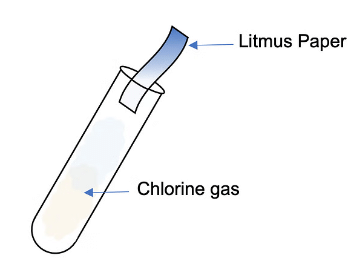
- If the gas produced at the cathode burns with a 'pop' when a sample is lit with a lighted splint, then the gas is hydrogen.
Diagram showing the test for hydrogen gas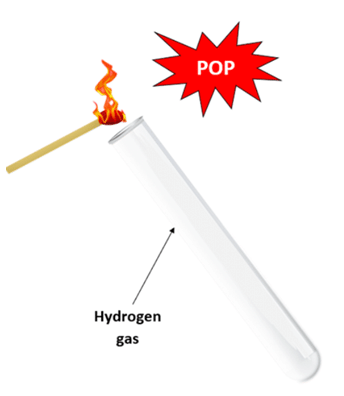
The document Electrolysis of Aqueous Sodium Chloride and Dilute Sulfuric Acid | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Electrolysis of Aqueous Sodium Chloride and Dilute Sulfuric Acid - Chemistry for GCSE/IGCSE - Class 10
| 1. What are the products formed at the electrodes during the electrolysis of brine? |  |
Ans. At the cathode, hydrogen gas is produced, while at the anode, chlorine gas is formed during the electrolysis of brine.
| 2. What happens during the electrolysis of dilute sulfuric acid? |  |
Ans. When dilute sulfuric acid is electrolyzed, hydrogen gas is produced at the cathode, while oxygen gas is formed at the anode.
| 3. How does electrolysis of aqueous sodium chloride differ from dilute sulfuric acid electrolysis? |  |
Ans. In the electrolysis of aqueous sodium chloride, chlorine gas is produced at the anode, whereas in dilute sulfuric acid electrolysis, oxygen gas is formed at the anode.
| 4. Why is it important to consider the electrolysis of brine and dilute sulfuric acid in Year 11 chemistry? |  |
Ans. Understanding the electrolysis of brine and dilute sulfuric acid is crucial in Year 11 chemistry as it helps students grasp key concepts related to electrochemistry and the production of useful gases.
| 5. What are some practical applications of the electrolysis of aqueous sodium chloride and dilute sulfuric acid? |  |
Ans. The electrolysis of aqueous sodium chloride is used in the production of chlorine and sodium hydroxide, while dilute sulfuric acid electrolysis is utilized in the generation of hydrogen gas for various industrial processes.
Related Searches




















