Eletrolysos of Aqueous Solutions | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Electrolysis of Aqueous Solutions
- Aqueous solutions always contain water (H2O).
- During the electrolysis of aqueous solutions, water molecules break down into H+ and OH- ions.
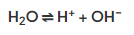
- These ions play a crucial role in the electrolysis process, considering their chemistry.
- The resulting electrolyte comprises ions from the compound and water.
- The discharge of ions at electrodes depends on the elements' relative reactivity.
- Different products are obtained from concentrated and dilute solutions of the same compound.
- For anions, the more concentrated ion tends to be discharged over the more dilute ion.
- Concentration impacts the discharge of ions during electrolysis.
- In the case of anions, the more concentrated ion takes precedence over the more dilute one.
Positive Electrode (anode)
- Negatively charged hydroxide (OH-) ions and non-metal ions are attracted to the positive electrode.
- If halide ions (chloride, bromide, iodide) and hydroxide ions are present, the halide ion is discharged at the anode. It loses electrons and forms a halogen (like chlorine, bromine, or iodine).
- In the absence of halide ions, hydroxide ions are discharged at the anode, losing electrons and forming oxygen gas.
- In both scenarios, the other negative ion remains in the solution.
- The concentration of the solution plays a role in which ion is discharged:
- In a concentrated halide solution undergoing electrolysis, the halogen forms at the anode.
- In a dilute halide solution undergoing electrolysis, oxygen is formed.
- For instance:
- In a concentrated solution of barium chloride, chlorine gas is produced at the anode because Cl- ions are discharged more readily than OH- ions.
- Conversely, in a dilute solution of barium chloride, oxygen is formed at the anode as only the OH- ions are discharged.
Negative Electrode (cathode)
- Positively charged H+ and metal ions are attracted to the negative electrode during electrolysis.
- Only one of these ions will gain electrons, leading to the production of either hydrogen gas or metal at the cathode.
- If the metal is higher than hydrogen in the reactivity series, hydrogen gas will be produced.
- Bubbling will be observed at the cathode due to the release of hydrogen gas.
- The more reactive metal ions will stay in the solution, causing the discharge of the ions of the less reactive metal.
- Therefore, unless the positive ions from the ionic compound are less reactive than hydrogen, hydrogen gas will be produced at the cathode.
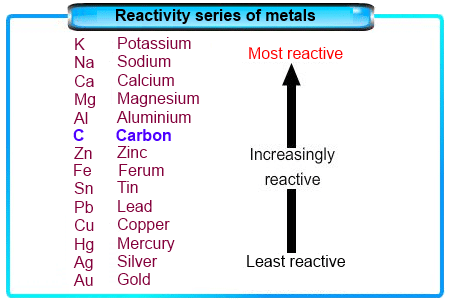
The Reactivity Series of Metals
The reactivity series of metals ranks metals in order of their reactivity. It includes hydrogen and carbon.
- Aqueous copper sulfate contains ions such as Cu²⁺, SO₄²⁻, H⁺, and OH⁻.
- Copper sulfate solution includes Cu²⁺, SO₄²⁻, H⁺, and OH⁻ ions.
Using Graphite Electrodes
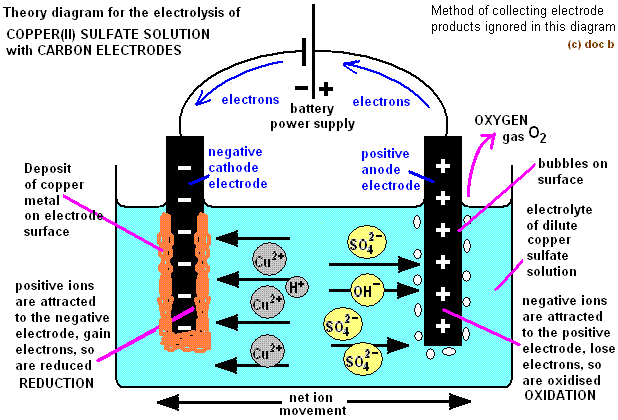
Apparatus for the electrolysis of copper(II) sulfate involves the use of inert graphite electrodes.
Product at the Cathode
- At the cathode, both Cu²⁺ and H⁺ ions are attracted, but the less reactive ion is discharged.
- In this case, copper is less reactive than hydrogen, resulting in the discharge of copper ions at the cathode.
- Copper ions gain electrons, get reduced, and form copper metal.
- The half equation for the reaction at the electrode can be represented.
Cu2+ + 2e- → Cu
Product at the Anode
- Both SO42- and OH- are attracted to the anode.
- OH- ions have a higher tendency to lose electrons compared to SO42-, resulting in their oxidation to form oxygen gas.
- The half equation for the reaction at the anode is: 4OH- → O2 + 2H2O + 4e-
Using Copper Electrodes
The apparatus used for the electrolysis of copper(II) sulfate involves active copper electrodes.
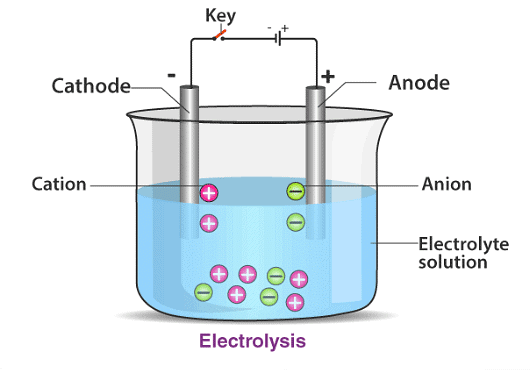
Observations at the Anode and Cathode
- The cathode gains mass while the anode loses mass.
- This occurs because copper atoms are oxidized at the anode, forming ions, while copper ions are reduced at the cathode, forming copper atoms.
- The gain in mass at the negative electrode equals the loss in mass at the positive electrode.
- This implies that the copper deposited on the negative electrode is the same as the copper ions lost from the positive electrode, maintaining a constant concentration of Cu2+ ions in the solution.
Products Formed for Common Aqueous Solutions
When electrolysis is performed on common aqueous solutions, various products are formed. Here is a table illustrating the products:
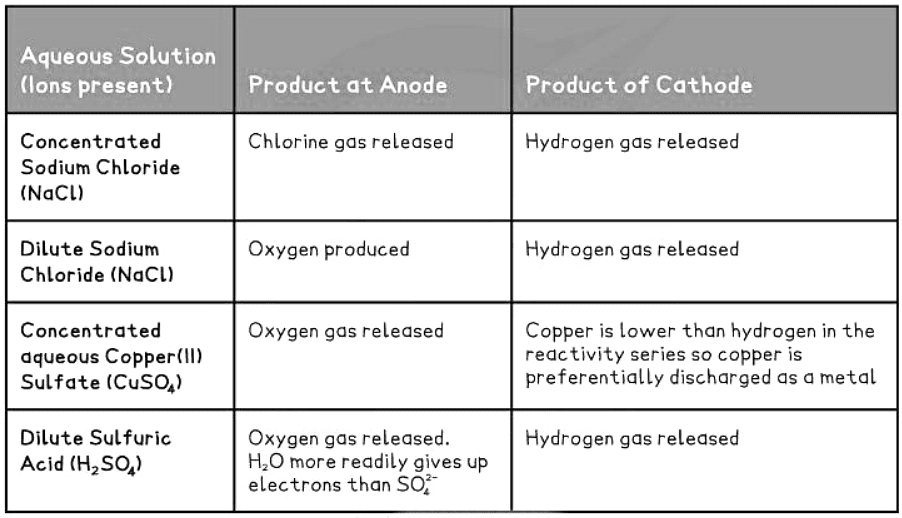
|
71 videos|147 docs|61 tests
|
FAQs on Eletrolysos of Aqueous Solutions - Chemistry for GCSE/IGCSE - Class 10
| 1. How does the electrolysis of aqueous solutions using copper electrodes differ from using other types of electrodes? |  |
| 2. What are the key factors that can influence the electrolysis of aqueous solutions with copper electrodes? |  |
| 3. Can the electrolysis of aqueous solutions with copper electrodes be used for industrial applications? |  |
| 4. How can the efficiency of the electrolysis process using copper electrodes be improved? |  |
| 5. Are there any safety considerations to keep in mind when performing electrolysis of aqueous solutions with copper electrodes? |  |















