Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Enthalpy Change & Activation Energy
Enthalpy Change & Activation Energy | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Enthalpy Change & Activation Energy
- Chemical reactions require atoms or particles to collide for them to occur within a system.
- Several factors influence collisions, including energy, orientation, and collision frequency.
- Each collision must possess a minimum energy threshold for successful reaction, termed activation energy (Ea).
- Activation energy varies among reactions, depending on the specific chemical species involved.
- Reactions with higher activation energies necessitate more initial energy input compared to those with lower activation energies.
- The exchange of thermal energy during a reaction is known as the enthalpy change (ΔH), which can be either positive (endothermic) or negative (exothermic) based on the reaction type.
Reaction Pathway Diagrams
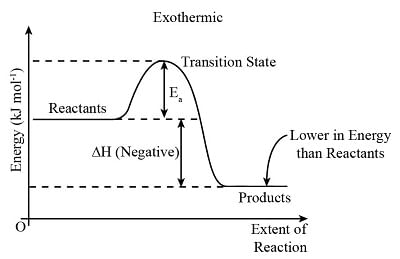
Exothermic Reactions
- Exothermic reactions release more energy than they absorb.
- Energy released during the formation of new bonds exceeds the energy needed to break bonds in the reactants.
- The energy change is negative as the products possess less energy than the reactants.
- Consequently, exothermic reactions are characterized by a negative ΔH value.
- The reaction pathway diagram illustrates the process of an exothermic reaction.
- The diagram represents the pathway for exothermic reactions:
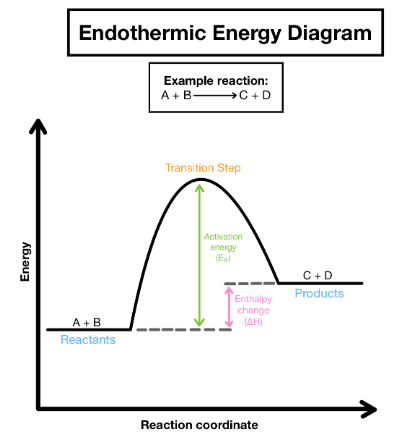
Endothermic Reactions
- Endothermic reactions absorb more energy to break bonds than they release to form new bonds.
- The energy change is positive because the products possess more energy than the reactants.
- Consequently, an endothermic reaction is characterized by a positive ΔH value, evident in energy level diagrams and calculations.
Exothermic Reactions:
- Release more energy than they absorb.
- Energy is released during bond formation compared to bond breaking in the reactants.
- The energy change is negative because products have lower energy levels than reactants.
- This results in a negative ΔH value for exothermic reactions.
The Reaction Pathway for Endothermic Reactions
- If more energy is required to break bonds than is released during bond formation, the reaction is termed endothermic overall.
- Endothermic reactions exhibit a positive energy change since products possess higher energy levels than reactants.
- A positive ΔH value characterizes endothermic reactions, evident in energy level diagrams and calculations.
- The reaction pathway diagram above illustrates endothermic reactions.
Question for Enthalpy Change & Activation EnergyTry yourself: What is activation energy (Ea) in a chemical reaction?View Solution
The document Enthalpy Change & Activation Energy | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
72 videos|162 docs|61 tests
|
FAQs on Enthalpy Change & Activation Energy - Chemistry for GCSE/IGCSE - Class 10
| 1. What is the relationship between enthalpy change and activation energy in a chemical reaction? |  |
Ans. Enthalpy change refers to the heat exchange during a chemical reaction, while activation energy is the energy required to initiate the reaction. The enthalpy change determines whether a reaction is exothermic or endothermic, while activation energy determines the rate at which the reaction occurs.
| 2. How are reaction pathway diagrams used to visualize the energy changes in a chemical reaction? |  |
Ans. Reaction pathway diagrams show the energy levels of reactants, transition states, and products during a reaction. They help visualize the activation energy required for the reaction to occur and the overall enthalpy change of the reaction.
| 3. How does the activation energy affect the speed of a chemical reaction? |  |
Ans. A higher activation energy barrier means that fewer molecules have enough energy to react, resulting in a slower reaction rate. Lowering the activation energy through catalysts can increase the reaction rate by providing an alternative pathway with a lower energy barrier.
| 4. Can a reaction be endothermic but have a low activation energy? |  |
Ans. Yes, a reaction can be endothermic (absorbing heat) but still have a low activation energy. The endothermic nature of the reaction indicates that the products have higher energy than the reactants, while the low activation energy allows the reaction to proceed relatively quickly.
| 5. How does the enthalpy change affect the stability of the products in a chemical reaction? |  |
Ans. The enthalpy change determines the overall energy difference between the reactants and products. A negative enthalpy change (exothermic reaction) results in lower energy products, making them more stable. In contrast, a positive enthalpy change (endothermic reaction) leads to higher energy products, which may be less stable.
Related Searches















