Grade 9 Exam > Grade 9 Notes > AP Chemistry > Physical and Chemical Change
Physical and Chemical Change | AP Chemistry - Grade 9 PDF Download
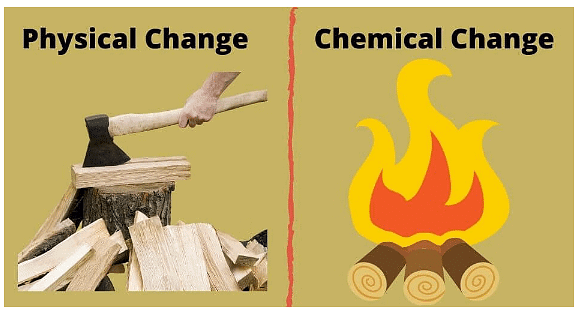
Physical Change
- Physical changes, like melting or evaporating, do not result in the formation of new chemical substances.
- These alterations are typically reversible and do not involve the creation of new compounds.
- Examples include forming mixtures from multiple substances or dissolving solutes in solvents.
- Such changes are categorized as physical because they do not yield new substances and are usually simple to undo or separate.
Question for Physical and Chemical ChangeTry yourself: Which of the following is an example of a physical change?View Solution
Chemical Change
- Chemical changes, known as chemical reactions, result in the creation of new substances with distinct properties from the initial reactants.
- Indications of such changes include alterations in color, the formation of a precipitate, or the release of gas bubbles.
- Reversing chemical reactions is often challenging due to the formation of new substances.
- Energy changes occur alongside chemical reactions, where energy can be released (exothermic) or absorbed (endothermic).
- The majority of chemical reactions release energy (exothermic), while only a minority absorb energy (endothermic).
Question for Physical and Chemical ChangeTry yourself: Which of the following is an indication of a chemical change?View Solution
The document Physical and Chemical Change | AP Chemistry - Grade 9 is a part of the Grade 9 Course AP Chemistry.
All you need of Grade 9 at this link: Grade 9
|
69 videos|92 docs|38 tests
|
FAQs on Physical and Chemical Change - AP Chemistry - Grade 9
| 1. What is a physical change? |  |
Ans. A physical change is a change in a substance that does not involve a change in its chemical composition. Examples include changes in state (solid to liquid), shape, size, or color.
| 2. Can physical changes be reversed? |  |
Ans. Yes, physical changes can typically be reversed by applying the appropriate conditions. For example, melting ice (solid to liquid) can be reversed by freezing it back (liquid to solid).
| 3. What is a chemical change? |  |
Ans. A chemical change is a change in a substance that results in the formation of new substances with different chemical properties. This change is usually irreversible.
| 4. How can you tell the difference between a physical and chemical change? |  |
Ans. One way to differentiate between physical and chemical changes is to observe if new substances are formed. If new substances are produced, it is a chemical change. If only the physical state or appearance of the substance changes, it is a physical change.
| 5. Can a substance undergo both physical and chemical changes? |  |
Ans. Yes, a substance can undergo both physical and chemical changes. For example, melting (physical change) followed by burning (chemical change) of a candle involves both types of changes.
Related Searches




















