Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Collision Theory
Collision Theory | Chemistry for GCSE/IGCSE - Class 10 PDF Download
What is Collision Theory?
- According to collision theory, for a reaction to occur:
- Particles must collide with one another.
- Collisions must possess adequate energy to initiate a reaction, enough to break existing bonds.
- The minimum energy required for colliding particles to react is termed the activation energy.
- Collisions that lead to a reaction are termed successful collisions:
- If the colliding particles possess sufficient energy (exceeding the activation energy), they react, resulting in a successful collision.
- Not all collisions lead to chemical reactions:
- Many collisions simply cause the particles to rebound off each other.
- Collisions that do not result in a reaction are termed unsuccessful collisions.
- Unsuccessful collisions occur when the colliding species lack the necessary energy to break bonds:
- They collide with energy below the activation energy threshold.
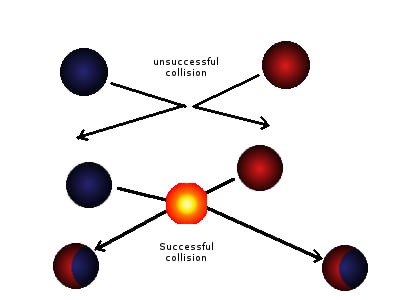 Diagram showing a successful and an unsuccessful collision
Diagram showing a successful and an unsuccessful collision
- Enhancing the number of successful collisions increases the proportion of reactant particles combining to form product molecules.
- The frequency of successful collisions hinges on:
- The density of particles within a given volume, as higher particle counts result in more frequent successful collisions.
- Collision occurrence rate, where a greater number of collisions per second leads to a higher frequency of successful collisions.
- Particle kinetic energy, where higher kinetic energy levels lead to more collisions surpassing the activation energy threshold, as faster-moving particles result in more frequent successful collisions.
- Activation energy levels, where higher activation energies lead to fewer collisions surpassing the threshold, resulting in fewer successful collisions.
- These factors collectively influence the reaction rate, determined by the rate of successful collisions per unit time.
Question for Collision TheoryTry yourself: What is necessary for a collision to result in a chemical reaction?View Solution
The document Collision Theory | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Collision Theory - Chemistry for GCSE/IGCSE - Class 10
| 1. What is Collision Theory? |  |
Ans. Collision Theory is a theory that explains how chemical reactions occur and the factors that affect their rates. It states that for a reaction to occur, particles must collide with enough energy and in the correct orientation.
| 2. What are the key components of Collision Theory? |  |
Ans. The key components of Collision Theory include the importance of collision frequency, the energy of collisions, and the orientation of colliding particles. These factors determine the likelihood of a successful reaction.
| 3. How does temperature affect Collision Theory? |  |
Ans. Temperature plays a crucial role in Collision Theory as it affects the kinetic energy of particles. Higher temperatures result in increased kinetic energy, leading to more frequent and energetic collisions, thus increasing the reaction rate.
| 4. Why is surface area important in Collision Theory? |  |
Ans. Surface area influences Collision Theory by affecting the number of particles available for collisions. A larger surface area provides more opportunities for collisions to occur, increasing the reaction rate.
| 5. How do catalysts impact Collision Theory? |  |
Ans. Catalysts lower the activation energy required for a reaction to occur, making it easier for particles to collide effectively. By providing an alternative pathway with lower energy requirements, catalysts enhance the rate of reactions without being consumed in the process.
Related Searches




















