Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Reversible Reactions
Reversible Reactions | Chemistry for GCSE/IGCSE - Class 10 PDF Download
| Table of contents |

|
| Reversible reactions |

|
| Chemical Equations for Reversible Reactions |

|
| Hydrated and Anhydrous Salts |

|
| Hydration of Cobalt(II) Chloride |

|
Reversible reactions
In reversible reactions, some key points to remember are:
- Completion of Reactions: Some reactions go to completion, where reactants are entirely used up to produce product molecules, and the reaction comes to a halt when all reactants are exhausted.
- Reversible Nature: Reversible reactions involve product molecules that can either react with each other or break down to reform the original reactant molecules.
- Bi-Directional Process: These reactions exhibit a dual nature, allowing them to proceed in two ways: the forward reaction, which results in the formation of products, and the reverse reaction, which leads to the regeneration of reactants.
Chemical Equations for Reversible Reactions
- Chemical equations for reversible reactions involve using two arrows to show forward and reverse reactions.
- Each arrow is depicted with half an arrowhead - one pointing right (→) for the forward reaction and the other pointing left (←) for the reverse reaction.
Example
- An illustration of a reversible reaction is the Haber process, where ammonia is produced from hydrogen and nitrogen.
N2 + 3H2 ⇌ 2NH3
Question for Reversible ReactionsTry yourself: What is a key characteristic of reversible reactions?View Solution
Hydrated and Anhydrous Salts
- Hydrated salts are salts that contain water of crystallization, influencing their molecular shape and color.
- Water of crystallization is the water that is stoichiometrically included in the structure of certain salts during the crystallization process.
- An illustration of this is seen in copper(II) sulfate, which crystallizes to form copper(II) sulfate pentahydrate, CuSO4 · 5H2O.
- Water of crystallization is denoted by a dot positioned between the salt molecule and the surrounding water molecules.
- Anhydrous salts are those that have undergone dehydration, usually through heating, resulting in the loss of their water of crystallization.
Dehydration of Hydrated Cobalt(II) Chloride
hydrated cobalt(II) chloride ⇌ anhydrous cobalt(II) chloride + water
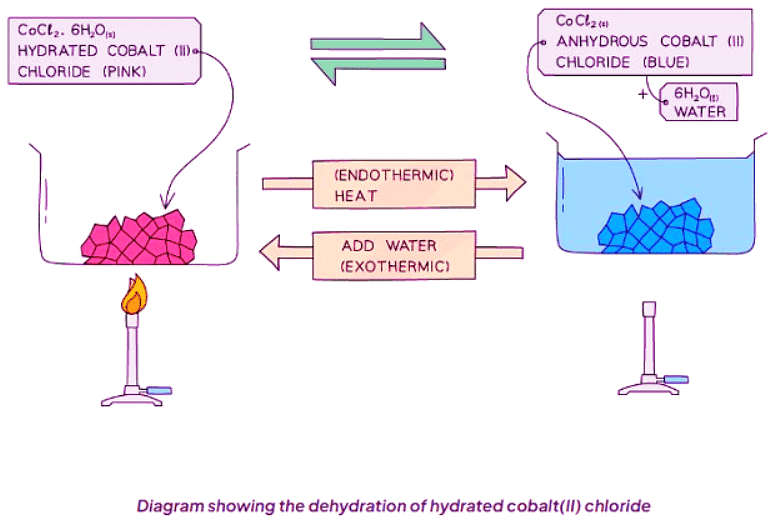
Hydration of Cobalt(II) Chloride
- Anhydrous blue cobalt(II) chloride crystals change to pink when added to water, a reversible reaction.
- Heating these pink crystals in a test tube causes them to revert to their original blue color as they lose water of crystallization.
- The pink crystals represent hydrated cobalt(II) chloride, containing water molecules within their structure.
- Heating hydrated cobalt(II) chloride causes it to lose its water of crystallization and transform into anhydrous cobalt(II) chloride.
- The chemical equation for this reaction is: CoCl2.6H2O (s) ⇌ CoCl2 (s) + 6H2O (l).
Question for Reversible ReactionsTry yourself: What is the difference between hydrated and anhydrous salts?View Solution
The document Reversible Reactions | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
Related Searches















