Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Haber Process
Haber Process | Chemistry for GCSE/IGCSE - Class 10 PDF Download
The Haber Process
The Haber process is crucial for the production of ammonia, a key component in various industries. Here's a breakdown of the process:
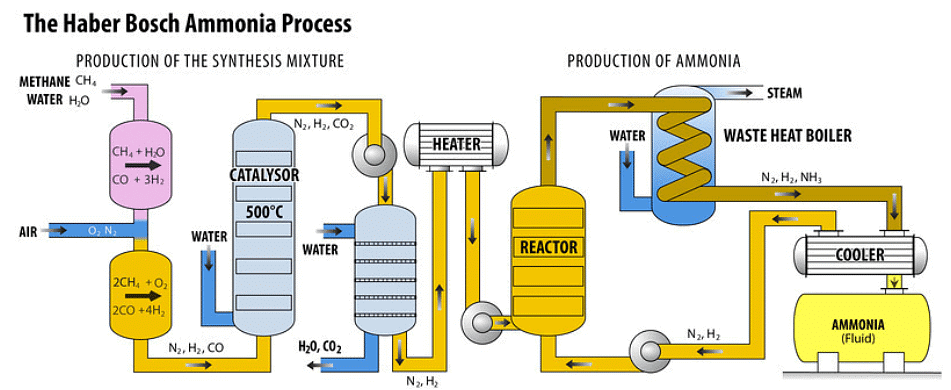
- Ammonia Production through the Haber Process: The Haber process is an exothermic reaction that involves the synthesis of ammonia in five distinct stages.
- Stage 1: Sourcing of Hydrogen and Nitrogen: Hydrogen (H2) is sourced from natural gas, while Nitrogen (N2) is obtained from the air. These gases are then directed into the compressor through pipes.
- Stage 2: Compression of Gases: Within the compressor, the gases are compressed to around 200 atmospheres, increasing their pressure significantly.
- Stage 3: Catalytic Conversion to Ammonia: The pressurized gases are introduced into a tank containing catalytic iron beds at a temperature of 450°C. In this environment, a portion of hydrogen and nitrogen reacts to produce ammonia.
The chemical equation representing this reaction is:
N2 (g) + 3H2 (g) ⟶ 2NH3 (g) - Stage 4: Unreacted hydrogen (H2) and nitrogen (N2) along with the produced ammonia move into a cooling tank where the ammonia is condensed into liquid form and then extracted for storage in pressurized containers.
- Stage 5: The hydrogen and nitrogen gases that were not converted in the reaction are cycled back into the system to begin the process anew.
Explaining the Conditions in the Haber Process
- Reaction conditions like temperature and pressure play a significant role in influencing how quickly a reaction proceeds.
- If a reaction is reversible, then changes in temperature and pressure impact the position of equilibrium. This often requires a balance between the reaction rate and the amount of product formed.
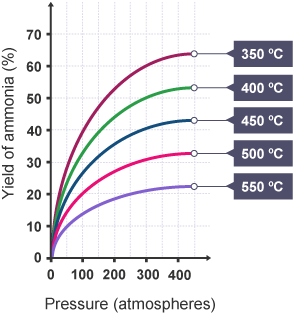
- The graph below demonstrates how altering temperature and pressure affects the yield of ammonia produced.
- When examining the graph, it is evident that increasing pressure leads to a higher yield at a given temperature.
- Conversely, decreasing temperature results in an increased yield, as shown by vertical lines moving upwards from the x-axis on the graph.
- Selecting the appropriate conditions depends on various factors, including economic, chemical, and practical considerations.
Conditions for Haber Process
Question for Haber ProcessTry yourself: What is the purpose of the Haber process?View Solution
Economic Considerations
- Like any business, companies in chemical manufacturing aim for profitability.
- A crucial aspect of industrial operations involves making strategic decisions about the design and location of manufacturing facilities.
- The availability and affordability of raw materials play a significant role and necessitate thorough analysis before decisions are made.
- In the Haber Process, raw materials like nitrogen, obtained from the air, and hydrogen, derived from natural gas, are both abundant and cost-effective to purify.
- If the extraction costs of raw materials are excessive or if they are unavailable, the process becomes economically unfeasible.
- Many industrial processes demand substantial heat and pressure, which can be financially burdensome to maintain.
- Energy costs associated with production are another critical consideration, which must be carefully evaluated alongside the raw materials issue.
Temperature: 450 ºC
- Higher temperatures favor the reverse reaction due to its endothermic nature, resulting in increased reactant production.
- Lower temperatures promote the forward reaction, as it's exothermic, leading to higher product yields.
- However, lower temperatures also mean slower reaction rates.
- Thus, 450 ºC serves as a compromise, balancing between higher product yields and quicker reaction times.
Pressure: 200 atm
- Lower pressure favors the reverse reaction as the system seeks to raise pressure by generating more gas molecules (4 gaseous reactant molecules), resulting in increased reactant yield.
- Conversely, higher pressure promotes the forward reaction as it aims to decrease pressure by producing fewer gas molecules (2 gaseous product molecules), leading to higher product yields.
- However, operating at high pressures poses safety risks and requires costly equipment.
- Therefore, a pressure of 200 atm serves as a compromise, ensuring a balance between safely and economically achieving a higher product yield.
Catalyst: Iron
- Catalysts don't alter the equilibrium position but accelerate the rate at which equilibrium is attained by facilitating both forward and backward reactions through a lower activation energy pathway.
- Consequently, the concentrations of reactants and products at equilibrium remain unaffected by the catalyst's presence.
- Catalysts expedite equilibrium attainment, aiding the reaction in reaching equilibrium faster.
- They enable achieving a satisfactory yield at lower temperatures by reducing the activation energy needed.
- Without catalysts, higher temperatures would be necessary, elevating costs and diminishing yields due to increased NH3 molecule decomposition.
Question for Haber ProcessTry yourself: What role does temperature play in the Haber Process?View Solution
The document Haber Process | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Haber Process - Chemistry for GCSE/IGCSE - Class 10
| 1. What are the key conditions required for the Haber Process to occur? |  |
Ans. The key conditions for the Haber Process include high pressure (around 200 atmospheres), high temperature (around 450 degrees Celsius), an iron catalyst, and a carefully controlled ratio of hydrogen and nitrogen gases.
| 2. How do the conditions in the Haber Process affect the yield of ammonia? |  |
Ans. The high pressure and temperature in the Haber Process help increase the rate of reaction, leading to a higher yield of ammonia. The iron catalyst also helps to speed up the reaction, further increasing the production of ammonia.
| 3. What economic considerations are important in the Haber Process? |  |
Ans. Economic considerations in the Haber Process include the cost of energy required to maintain high pressure and temperature, the cost of the catalyst, and the overall efficiency of the process in producing ammonia.
| 4. Why is the Haber Process considered crucial for agricultural production? |  |
Ans. The ammonia produced in the Haber Process is a key component in the production of fertilizers, which are essential for promoting plant growth and increasing crop yields in agriculture.
| 5. How does the Haber Process contribute to sustainability in agriculture? |  |
Ans. By providing a reliable and efficient method for producing ammonia, the Haber Process helps ensure a steady supply of fertilizers for agricultural use, ultimately supporting sustainable farming practices and food production.
Related Searches















